General Information About Gastrointestinal Stromal Tumors (GISTs)
Incidence
GISTs comprise less than 1% of all gastrointestinal (GI) tumors but are the most common mesenchymal tumors of the GI tract.[1,2,3] There are estimated to be over 6,000 new GIST cases per year in the United States, with an age-adjusted yearly incidence of 6.78 per million from 2001 to 2011. GISTs can affect patients of all ages but are most predominant in older adults (median age, 65–69 years).[4,5] Globally, GISTs affect men and women with equal frequency. Geographically, GISTs are most prevalent in China (Shanghai), Taiwan, Korea, and Norway.[5] In the United States, GISTs are more commonly diagnosed in Black Americans (13.7 per million) and Asian or Pacific Islander Americans (11 per million) than in White Americans (6.5 per million).[4]
The true incidence is not known, in part, because small indolent GISTs (i.e., <1 cm) are either not clinically apparent or are not included in cancer registries.[5,6,7]
Most GISTs are sporadic, but there are rare familial forms associated with neurofibromatosis type 1 (NF1) or heritable mutations in KIT and SDH.[2,3] GISTs rarely affect children and young adults (<1% of cases), with a median age of 15 years in cases that are nearly always associated with an underlying genetic predisposition.[8,9] For more information, see Childhood Gastrointestinal Stromal Tumors Treatment.
Clinical Presentation
GISTs can occur anywhere along the GI tract, but most often are found in the stomach or small intestine. The American Joint Committee on Cancer (AJCC) Cancer Staging Manual lists the following approximate distributions:[10]
- Stomach (60%).
- Small intestine, jejunum and ileum (30%).
- Duodenum (5%).
- Rectum (3%).
- Colon (1%).
- Esophagus (<1%).
- Disseminated tumors without a known primary (rare).
- Omentum/mesentery (rare).
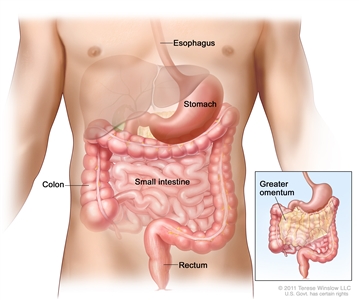
Gastrointestinal stromal tumors (GISTs) may be found anywhere in or near the gastrointestinal tract.
GISTs range in size from less than 1 cm to more than 40 cm, with an average size of approximately 5 cm when diagnosed clinically. They typically arise within the muscle wall of the GI tract.[11] Small GISTs may form solid subserosal, intramural, or, less frequently, polypoid intraluminal masses. Large tumors tend to form external masses attached to the outer aspect of the bowel wall involving the muscular layers.[11]
The clinical presentation of patients with GISTs varies depending on the following:[12,13]
- Anatomical location.
- Tumor size.
- Rate of tumor growth.
The signs and symptoms of GISTs include the following:
- GI bleeding (most common presentation), which may be acute (melena or hematemesis) or chronic, resulting in anemia.
- Acute tumor rupture.
- GI obstruction.
- Pain.
- Dysphagia.
- Early satiety.
Smaller lesions may be found incidentally during surgery, radiological studies, or endoscopy. The natural history of these incidental tumors and the frequency of progression to symptomatic disease are unknown. There may be a substantial reservoir of small GISTs that do not progress to symptomatic stages.
Common sites of metastasis include the liver and peritoneal dissemination within the abdominal cavity. In adults, lymph node involvement and spread to the lungs or other extra-abdominal sites is unusual.[14]
Rare paraneoplastic consumptive hypothyroidism (from overexpression of a thyroid-inactivating enzyme) has been reported in a few patients.[15]
Pediatric GISTs are typically associated with germline SDH loss. The clinical behavior is distinct with typically a gastric location, more indolent course, multifocal presentation, and lymph node metastases. Germline SDH loss is also associated with hereditary kidney cancer, paragangliomas, and other tumors.[16,17]
Diagnostic Evaluation
GISTs should be included in the differential diagnosis of any intra-abdominal nonepithelial malignancy. Standard diagnostic interventions may include the following:[12]
- Computed tomography (CT).
- Magnetic resonance imaging (MRI).
- Positron emission tomography (PET).
- Endoscopy.
Endoscopic ultrasound with fine-needle aspiration (FNA) biopsy is useful in the diagnosis of GISTs in the upper GI tract, as most tumors arise below the mucosal layer and grow in an endophytic fashion. Endoscopic ultrasound–guided FNA biopsy is preferred to percutaneous biopsy, given the risk of tumor hemorrhage and peritoneal dissemination.[12,18,19] For localized resectable GISTs with classic imaging findings, some surgeons proceed directly to surgery without biopsy.
Prognosis
Prognostic factors for nonmetastatic GISTs include the following:
- Mitotic index.
- Tumor size.
- Tumor location (gastric, nongastric, rectal).
- Tumor rupture.
- Imaging characteristics.
Approximately 20% to 25% of gastric GISTs and 40% to 50% of small intestinal GISTs are clinically aggressive.[13,20] It is estimated that approximately 10% to 25% of patients present with metastatic disease.[14,20] For nonmetastatic GISTs, the key parameters that impact the risk of recurrence or metastasis include mitotic index (mitoses per 50 high-power fields), tumor size, and tumor location (see Table 1).[11,21,22,23,24,25]
It is also recognized that tumor rupture markedly worsens recurrence-free survival.[26,27,28] In addition, tumor appearance on CT imaging may predict recurrence risk. Tumors with higher metastatic risk include lobulated or heterogeneously enhancing tumors, as well as those with mesenteric fat infiltration, ulceration, regional lymphadenopathy, or exophytic growth.[29,30,31,32]
Table 1. Risk Assessment of Gastric GISTs by Tumor Size and Mitotic Indexa
| Mitotic Index (mitoses/HPF) |
Size (cm) |
Metastasis Rate (%) |
Risk of Progressive Disease |
| GISTs = gastrointestinal stromal tumors; HPF = high-power field. |
|
a Adapted from Miettinen et al.[25]and Laurini et al.[33] |
| ≤5 per 50 |
≤2 |
0 |
None |
| >2 to ≤5 |
1.9 |
Very low |
| >5 to ≤10 |
3.6 |
Low |
| >10 |
12 |
Moderate |
| >5 per 50 |
≤2 |
0 |
None |
| >2 to ≤5 |
16 |
Moderate |
| >5 to ≤10 |
55 |
High |
| >10 |
86 |
High |
Table 2. Risk Assessment of Nongastric GISTs by Tumor Size and Mitotic Indexa
| Mitotic Index (mitoses/HPF) |
Size (cm) |
Metastasis Rate (%) |
Risk of Progressive Disease |
| GISTs = gastrointestinal stromal tumors; HPF = high-power field. |
|
a Adapted from Miettinen et al.[25]and Laurini et al.[33] |
| ≤5 per 50 |
≤2 |
0 |
None |
| >2 to ≤5 |
1.9–8.5 |
Low |
| >5 to ≤10 |
24 |
Insufficient data–Moderate |
| >10 |
34–52 |
High |
| >5 per 50 |
≤2 |
50–54 |
Insufficient data–High |
| >2 to ≤5 |
50–73 |
High |
| >5 to ≤10 |
85 |
High |
| >10 |
71–90 |
High |
Follow-up
Response to therapy
CT, fluorine F 18-fludeoxyglucose (18F-FDG) PET, and MRI are used to monitor the effects of systemic therapy in patients with unresectable, metastatic, or recurrent disease.[34]
A baseline PET should be performed before tyrosine kinase inhibitor (TKI) therapy in patients who will be monitored for response with 18F-FDG PET. PET imaging may detect the activity of imatinib in GISTs much earlier than CT imaging, with decreased tumor avidity detected as soon as 24 hours after the first dose. Thus, PET may be a useful diagnostic modality for the very early assessment of response to imatinib therapy and for detecting resistance to TKIs.[12]
Surveillance for metastatic or recurrent disease
The optimal modality and frequency for surveillance of metastatic or recurrent disease in patients who have undergone GIST resection has not been studied. Based on the likelihood of recurrence, follow-up recommendations are derived from expert opinion and clinical judgment.
For surgically treated patients with localized disease, routine follow-up schedules may differ across institutions and depend on the risk status of the tumor.[35] Abdominal/pelvic imaging may be performed every 3 to 6 months, but very low-risk lesions may not need to be imaged this frequently.[35]
References:
-
Judson I, Demetri G: Advances in the treatment of gastrointestinal stromal tumours. Ann Oncol 18 (Suppl 10): x20-4, 2007.
-
Miettinen M, Lasota J: Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 438 (1): 1-12, 2001.
-
Miettinen M, Sarlomo-Rikala M, Lasota J: Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 30 (10): 1213-20, 1999.
-
Ma GL, Murphy JD, Martinez ME, et al.: Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev 24 (1): 298-302, 2015.
-
Søreide K, Sandvik OM, Søreide JA, et al.: Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 40: 39-46, 2016.
-
Kawanowa K, Sakuma Y, Sakurai S, et al.: High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol 37 (12): 1527-35, 2006.
-
Agaimy A, Wünsch PH, Hofstaedter F, et al.: Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol 31 (1): 113-20, 2007.
-
Benesch M, Wardelmann E, Ferrari A, et al.: Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatr Blood Cancer 53 (7): 1171-9, 2009.
-
Joensuu H, Hohenberger P, Corless CL: Gastrointestinal stromal tumour. Lancet 382 (9896): 973-83, 2013.
-
Gastrointestinal stromal tumor. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 523–9.
-
Corless CL, Heinrich MC: Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 3: 557-86, 2008.
-
Casali PG, Dei Tos AP, Gronchi A: Gastrointestinal stromal tumor. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, et al., eds.: DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. 11th ed. Wolters Kluwer, 2019, pp 895-906.
-
Miettinen M, Lasota J: Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130 (10): 1466-78, 2006.
-
DeMatteo RP, Lewis JJ, Leung D, et al.: Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231 (1): 51-8, 2000.
-
Maynard MA, Marino-Enriquez A, Fletcher JA, et al.: Thyroid hormone inactivation in gastrointestinal stromal tumors. N Engl J Med 370 (14): 1327-34, 2014.
-
Janeway KA, Pappo A: Treatment guidelines for gastrointestinal stromal tumors in children and young adults. J Pediatr Hematol Oncol 34 (Suppl 2): S69-72, 2012.
-
Miettinen M, Lasota J, Sobin LH: Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol 29 (10): 1373-81, 2005.
-
Nickl NJ: Gastrointestinal stromal tumors: new progress, new questions. Curr Opin Gastroenterol 20 (5): 482-7, 2004.
-
Vander Noot MR, Eloubeidi MA, Chen VK, et al.: Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 102 (3): 157-63, 2004.
-
Joensuu H: Gastrointestinal stromal tumor (GIST). Ann Oncol 17 (Suppl 10): x280-6, 2006.
-
Miettinen M, Sobin LH, Lasota J: Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29 (1): 52-68, 2005.
-
Miettinen M, Makhlouf H, Sobin LH, et al.: Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 30 (4): 477-89, 2006.
-
Miettinen M, Kopczynski J, Makhlouf HR, et al.: Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol 27 (5): 625-41, 2003.
-
Miettinen M, Furlong M, Sarlomo-Rikala M, et al.: Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol 25 (9): 1121-33, 2001.
-
Miettinen M, Lasota J: Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23 (2): 70-83, 2006.
-
Hohenberger P, Ronellenfitsch U, Oladeji O, et al.: Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 97 (12): 1854-9, 2010.
-
Hølmebakk T, Bjerkehagen B, Boye K, et al.: Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg 103 (6): 684-691, 2016.
-
Joensuu H: Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39 (10): 1411-9, 2008.
-
Chun HJ, Byun JY, Chun KA, et al.: Gastrointestinal leiomyoma and leiomyosarcoma: CT differentiation. J Comput Assist Tomogr 22 (1): 69-74, 1998 Jan-Feb.
-
Levy AD, Remotti HE, Thompson WM, et al.: Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics 23 (2): 283-304, 456; quiz 532, 2003 Mar-Apr.
-
Ghanem N, Altehoefer C, Furtwängler A, et al.: Computed tomography in gastrointestinal stromal tumors. Eur Radiol 13 (7): 1669-78, 2003.
-
Burkill GJ, Badran M, Al-Muderis O, et al.: Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology 226 (2): 527-32, 2003.
-
Laurini JA, et al.: Protocol For the Examination of Resection Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Version 4.2.0.0. College of American Pathologists, 2021. Available online. Last accessed October 18, 2022.
-
Demetri GD, Benjamin RS, Blanke CD, et al.: NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5 (Suppl 2): S1-29; quiz S30, 2007.
-
Casali PG, Jost L, Reichardt P, et al.: Gastrointestinal stromal tumors: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 19 (Suppl 2): ii35-8, 2008.
Cellular and Molecular Classification of GISTs
Gastrointestinal stromal tumors (GISTs) appear to originate from interstitial cells of Cajal (ICC) or their stem cell-like precursors.[1,2,3,4] ICC are pacemaker-like intermediates between the gastrointestinal (GI) autonomic nervous system and smooth muscle cells regulating GI motility and autonomic nerve function.[5,6] ICC are located around the myenteric plexus and the muscularis propria throughout the GI tract. ICC or their stem cell-like precursors can differentiate into smooth muscle cells if KIT signaling is disrupted.[7]
GISTs are composed of spindle cells (70%), epithelioid cells (20%), or mixed spindle and epithelioid cells (10%).[8] The histological patterns range from bland-appearing tumors with very low mitotic activity to very aggressive-appearing patterns.[9]
Approximately 85% of GISTs contain oncogenic mutations in one of two receptor tyrosine kinases (RTKs):[10,11]
Constitutive activation of either of these RTKs plays a central role in the pathogenesis of GISTs.[1,12] Tumors without detectable KIT or PDGFRA mutations account for 12% to 15% of all GISTs. Less than 5% of GISTs occur in the setting of syndromic diseases, such as neurofibromatosis type 1 (NF1), Carney triad syndrome (SDH deletion), and other familial diseases.[10,13,14,15]
Approximately 95% of GISTs are positive for the CD117 antigen, an epitope of KIT RTK expressed by ICC.[10] However, CD117 immunohistochemistry (IHC) is not specific for GISTs and can be seen in other mesenchymal, neural, and neuroendocrine neoplasms.[10] IHC staining for DOG1 helps distinguish GISTs from other mesenchymal tumors, particularly those that are KIT negative.[10,16,17,18]
Subtypes of GISTs include the following:
-
KIT-mutant GISTs. Approximately 80% of all GISTs contain a mutation in the KIT gene that results in constitutive activation.[10] The KIT gene maps to 4q12-13, in the vicinity of genes encoding the RTKs PDGFRA and VEGFR2.[19] Mutations in five different KIT exons have been observed in GISTs: exon 11 (67%), exon 9 (10%), and exons 8, 13, and 17 (3%).[10,20] Typically, GISTs are heterozygous for a particular mutation, but loss of the remaining wild-type KIT allele occurs in approximately 8% to 15% of tumors and may be associated with malignant progression.[20,21,22]KIT mutation variants exhibit distinct anatomical distributions: exon 8 (small bowel), exon 9 (small bowel, colon), and exons 11, 13, and 17 (all sites).[10]KIT-mutant tumors express PKC theta and DOG1, a distinguishing feature of mesenchymal tumors.[17,18,23]
-
PDGFRA-mutant GISTs. Approximately 5% to 8% of GISTs harbor a mutation in PDGFRA, a close homolog of KIT with similar extracellular and cytoplasmic domains.[12]PDGFRA-mutant GISTs may differ from KIT-mutant GISTs in a number of ways, including a marked predilection for the stomach, epithelioid morphology, myxoid stroma, nuclear pleomorphism, and variable expression of CD117.[23,24,25,26,27,28] As with KIT-mutant GISTs, PDGFRA-mutant tumors express PKC theta and DOG1.[17,18,24]PDGFRA mutation most commonly occurs in exon 18 (80%–90%), and can be either a D842V (62%) or non-D842V (27%) mutation. PDGFRA D842V mutations confer resistance to imatinib therapy.[29]
-
KIT-negative GISTs. In approximately 5% of GISTs, IHC for CD117 is completely negative or uncertain. In these instances, IHC may lack sufficient sensitivity to detect small amounts of mutant kinase.[10] Approximately 30% of these tumors harbor PDGFRA gene mutations while more than one-half have KIT mutations.[10,24,25,28]
-
KIT/PDGFRA wild-type GISTs. The so-called wild-type GISTs comprise approximately 12% to 15% of all GISTs. In these tumors, no detectable mutations have been identified in either KIT or PDGFRA. Many of these tumors are SDH-deficient or associated with NF1.
- SDH-deficient GISTs are characterized by loss-of-function of one of more enzymes within the SDH family (SDHA–D, collectively termed SDHx) either by mutation, such as in Carney-Stratakis syndrome, or epigenetic silencing, such as in Carney triad (gastric epithelioid GISTs, extra-adrenal paraganglioma, and pulmonary chondroma). SDH-deficient GISTs can be identified with IHC by an absence of SDHB. SDH-deficient GISTs are generally found in younger patients, are typically multifocal, and are located in the stomach. They also tend to have an indolent course and are poorly responsive to tyrosine kinase inhibitor therapy.[13,14,15,30]
- NF1-related GISTs have a propensity for multicentricity within the GI tract and spindle cell morphology. They are typically positive for the CD117 antigen but do not harbor KIT or PDGFRA mutations.[13] The clinical course is typically indolent.
- Other mutations seen in KIT/PDGFRA wild-type GISTs include BRAF V600E [31,32] and NTRK.[33]
-
Familial GISTs. Approximately two dozen kindreds with heritable mutations in KIT or PDGFRA have been identified. Penetrance in these kindreds is high, with most affected members developing one or more GISTs by middle age. However, in many patients, the tumors follow a benign course.[10]
References:
-
Hirota S, Isozaki K, Moriyama Y, et al.: Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279 (5350): 577-80, 1998.
-
Kindblom LG, Remotti HE, Aldenborg F, et al.: Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 152 (5): 1259-69, 1998.
-
Wang L, Vargas H, French SW: Cellular origin of gastrointestinal stromal tumors: a study of 27 cases. Arch Pathol Lab Med 124 (10): 1471-5, 2000.
-
Sircar K, Hewlett BR, Huizinga JD, et al.: Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol 23 (4): 377-89, 1999.
-
Maeda H, Yamagata A, Nishikawa S, et al.: Requirement of c-kit for development of intestinal pacemaker system. Development 116 (2): 369-75, 1992.
-
Huizinga JD, Thuneberg L, Klüppel M, et al.: W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373 (6512): 347-9, 1995.
-
Torihashi S, Nishi K, Tokutomi Y, et al.: Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology 117 (1): 140-8, 1999.
-
Corless CL, Fletcher JA, Heinrich MC: Biology of gastrointestinal stromal tumors. J Clin Oncol 22 (18): 3813-25, 2004.
-
Gastrointestinal stromal tumor. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 523–9.
-
Corless CL, Heinrich MC: Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 3: 557-86, 2008.
-
Miettinen M, Lasota J: Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130 (10): 1466-78, 2006.
-
Heinrich MC, Corless CL, Duensing A, et al.: PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299 (5607): 708-10, 2003.
-
Andersson J, Sihto H, Meis-Kindblom JM, et al.: NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol 29 (9): 1170-6, 2005.
-
Agaimy A, Pelz AF, Corless CL, et al.: Epithelioid gastric stromal tumours of the antrum in young females with the Carney triad: a report of three new cases with mutational analysis and comparative genomic hybridization. Oncol Rep 18 (1): 9-15, 2007.
-
Carney JA: Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc 74 (6): 543-52, 1999.
-
Blay P, Astudillo A, Buesa JM, et al.: Protein kinase C theta is highly expressed in gastrointestinal stromal tumors but not in other mesenchymal neoplasias. Clin Cancer Res 10 (12 Pt 1): 4089-95, 2004.
-
Duensing A, Joseph NE, Medeiros F, et al.: Protein Kinase C theta (PKCtheta) expression and constitutive activation in gastrointestinal stromal tumors (GISTs). Cancer Res 64 (15): 5127-31, 2004.
-
West RB, Corless CL, Chen X, et al.: The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 165 (1): 107-13, 2004.
-
Stenman G, Eriksson A, Claesson-Welsh L: Human PDGFA receptor gene maps to the same region on chromosome 4 as the KIT oncogene. Genes Chromosomes Cancer 1 (2): 155-8, 1989.
-
Heinrich MC, Corless CL, Demetri GD, et al.: Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21 (23): 4342-9, 2003.
-
O'Riain C, Corless CL, Heinrich MC, et al.: Gastrointestinal stromal tumors: insights from a new familial GIST kindred with unusual genetic and pathologic features. Am J Surg Pathol 29 (12): 1680-3, 2005.
-
Antonescu CR, Besmer P, Guo T, et al.: Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 11 (11): 4182-90, 2005.
-
Wasag B, Debiec-Rychter M, Pauwels P, et al.: Differential expression of KIT/PDGFRA mutant isoforms in epithelioid and mixed variants of gastrointestinal stromal tumors depends predominantly on the tumor site. Mod Pathol 17 (8): 889-94, 2004.
-
Debiec-Rychter M, Wasag B, Stul M, et al.: Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J Pathol 202 (4): 430-8, 2004.
-
Medeiros F, Corless CL, Duensing A, et al.: KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 28 (7): 889-94, 2004.
-
Sakurai S, Hasegawa T, Sakuma Y, et al.: Myxoid epithelioid gastrointestinal stromal tumor (GIST) with mast cell infiltrations: a subtype of GIST with mutations of platelet-derived growth factor receptor alpha gene. Hum Pathol 35 (10): 1223-30, 2004.
-
Wardelmann E, Hrychyk A, Merkelbach-Bruse S, et al.: Association of platelet-derived growth factor receptor alpha mutations with gastric primary site and epithelioid or mixed cell morphology in gastrointestinal stromal tumors. J Mol Diagn 6 (3): 197-204, 2004.
-
Pauls K, Merkelbach-Bruse S, Thal D, et al.: PDGFRalpha- and c-kit-mutated gastrointestinal stromal tumours (GISTs) are characterized by distinctive histological and immunohistochemical features. Histopathology 46 (2): 166-75, 2005.
-
Corless CL, Schroeder A, Griffith D, et al.: PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 23 (23): 5357-64, 2005.
-
Boikos SA, Pappo AS, Killian JK, et al.: Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2 (7): 922-8, 2016.
-
Agaram NP, Wong GC, Guo T, et al.: Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer 47 (10): 853-9, 2008.
-
Hostein I, Faur N, Primois C, et al.: BRAF mutation status in gastrointestinal stromal tumors. Am J Clin Pathol 133 (1): 141-8, 2010.
-
Atiq MA, Davis JL, Hornick JL, et al.: Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: a clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST). Mod Pathol 34 (1): 95-103, 2021.
Stage Information for GISTs
A formal staging system for gastrointestinal stromal tumors (GISTs) is available from the American Joint Committee on Cancer (AJCC) Staging Manual. In practice, however, AJCC staging is not routinely implemented when risk assessment is determined by the clinical features noted in the Prognosis section.[1]
References:
-
Gastrointestinal stromal tumor. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 523–9.
Treatment Option Overview for GISTs
The management of patients with gastrointestinal stromal tumors (GISTs) is a multidisciplinary effort involving close collaboration between pathologists, medical oncologists, surgeons, and imaging experts.[1]
Surgery
Surgical resection is the primary treatment modality for the following types of patients:[2][Level of evidence C2]
- Those with primary GISTs who do not have evidence of metastasis.
- Those with tumors that are technically resectable (e.g., GISTs that do not require a formal gastrectomy, pancreatectomy, or other major organ resection) if the risks of morbidity are acceptable.
Endoscopic surveillance is an option for patients with tumors measuring 2 cm or smaller with a mitotic index of 5 or less per 50 high-power fields. The low rates of progression and metastasis in these tumors make endoscopic surveillance viable in place of surgical resection.[3]
The goal of surgery is complete gross resection with an intact pseudocapsule and negative microscopic margins.[4] Because GISTs are generally encapsulated and relatively less infiltrative than other malignancies, wide excision is not necessary. Lymphadenectomy is typically unnecessary, given that lymph node metastasis is rare with GISTs. However, lymphadenectomy should be considered in patients with SDH-deficient GISTs and pathologically enlarged lymph nodes.
If anatomically feasible, laparoscopic surgery is increasingly performed instead of laparotomy. Reports demonstrate lower rates of recurrence, shorter hospital stays, and lower morbidity.[5,6,7,8]
Neoadjuvant imatinib therapy can be given to patients with large tumors or difficult-to-access GISTs that are considered marginally resectable. Significant tumor shrinkage is often seen with targeted therapy, so this approach can potentially avoid major organ resection, or enable organ-sparing surgery. Genetic sequencing may be considered to identify sensitive or resistant mutations prior to neoadjuvant imatinib therapy.
For patients with oligometastatic recurrences (e.g., isolated intra-abdominal implants or solitary liver lesions), surgical resection may be used in conjunction with tyrosine kinase inhibitors (TKIs).[9,10][Level of evidence C1] This should only be considered after multidisciplinary consultation.
Chemotherapy
There is universal agreement that standard chemotherapy has no role in the primary therapy of GISTs.[4,11,12]
Before the advent of molecularly targeted therapy with TKIs, efforts to treat GISTs with conventional cytotoxic chemotherapy were essentially futile.[1] The extreme resistance of GISTs to chemotherapy may be partly caused by the increased expression of P-glycoprotein, the product of the MDR-1 gene, and MRP1, which are cellular efflux pumps that may prevent chemotherapeutic agents from reaching therapeutic intracellular concentrations in GIST cells.[1,13]
Tyrosine Kinase Inhibitor (TKI) Therapy
TKIs work by inhibiting aberrantly functioning KIT or PDGFRA receptor tyrosine kinases and inducing rapid reduction in tumor growth. TKI therapy is indicated for patients with unresectable, borderline resectable, metastatic, or recurrent GISTs. It is also indicated as adjuvant therapy for patients with GISTs at high risk of recurrence.
The TKI imatinib mesylate is used as first-line therapy for most patients with KIT- and PDGFRA-mutant GISTs.[14] For patients with GISTs characterized by a PDGFRA D842V mutation, avapritinib is used as first-line therapy, given the high clinical benefit and imatinib-resistance in this subtype.[15] Other TKI agents approved for subsequent lines of therapy in patients with KIT/PDGFRA-mutant GISTs include sunitinib, regorafenib, and ripretinib. Additional TKI agents that are occasionally given include nilotinib, sorafenib, and pazopanib.
Imatinib is not typically given to patients with KIT/PDGFRA wild-type GISTs (i.e., SDH-deficient or neurofibromatosis type 1 [NF1]-related GISTs) because of high rates of resistance. Other TKIs (i.e., sunitinib or regorafenib) may have some activity, but most patients are recommended to consider enrolling in clinical trials, if eligible.
For more information on the efficacy, safety, and management of toxicity of imatinib, or additional agents in the setting of imatinib resistance or intolerance, see the Treatment of Resectable Primary GISTs, Treatment of Unresectable Primary GISTs, and Treatment of Metastatic or Recurrent GISTs sections.
Radiation Therapy
Radiation therapy rarely has a role in the management of patients with GISTs. It may occasionally be used for palliation of painful metastases or for patients with unresectable bleeding tumors.[1]
References:
-
Casali PG, Dei Tos AP, Gronchi A: Gastrointestinal stromal tumor. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, et al., eds.: DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. 11th ed. Wolters Kluwer, 2019, pp 895-906.
-
Judson I, Demetri G: Advances in the treatment of gastrointestinal stromal tumours. Ann Oncol 18 (Suppl 10): x20-4, 2007.
-
Miettinen M, Sobin LH, Lasota J: Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29 (1): 52-68, 2005.
-
Demetri GD, Benjamin RS, Blanke CD, et al.: NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5 (Suppl 2): S1-29; quiz S30, 2007.
-
Huguet KL, Rush RM, Tessier DJ, et al.: Laparoscopic gastric gastrointestinal stromal tumor resection: the mayo clinic experience. Arch Surg 143 (6): 587-90; discussion 591, 2008.
-
Otani Y, Furukawa T, Yoshida M, et al.: Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery 139 (4): 484-92, 2006.
-
Novitsky YW, Kercher KW, Sing RF, et al.: Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 243 (6): 738-45; discussion 745-7, 2006.
-
Chen K, Zhou YC, Mou YP, et al.: Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg Endosc 29 (2): 355-67, 2015.
-
Kanda T, Masuzawa T, Hirai T, et al.: Surgery and imatinib therapy for liver oligometastasis of GIST: a study of Japanese Study Group on GIST. Jpn J Clin Oncol 47 (4): 369-372, 2017.
-
Pawlik TM, Vauthey JN, Abdalla EK, et al.: Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 141 (6): 537-43; discussion 543-4, 2006.
-
Demetri GD, von Mehren M, Blanke CD, et al.: Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347 (7): 472-80, 2002.
-
Edmonson JH, Marks RS, Buckner JC, et al.: Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest 20 (5-6): 605-12, 2002.
-
Plaat BE, Hollema H, Molenaar WM, et al.: Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: differences in clinical outcome and expression of multidrug resistance proteins. J Clin Oncol 18 (18): 3211-20, 2000.
-
Blanke CD, Demetri GD, von Mehren M, et al.: Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26 (4): 620-5, 2008.
-
Heinrich MC, Jones RL, von Mehren M, et al.: Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol 21 (7): 935-946, 2020.
Treatment of Resectable Primary GISTs
Treatment Options for Resectable Primary GISTs
Treatment options for resectable primary gastrointestinal stromal tumors (GISTs) include the following:
- Surgery.
- Postoperative adjuvant tyrosine kinase inhibitor (TKI) therapy.
Surgery
All GISTs measuring 2 cm or larger are typically surgically resected. The management of incidentally encountered GISTs measuring smaller than 2 cm remains controversial. There is no evidence for re-excision in patients with a complete resection of all macroscopic disease but microscopically positive margins. Watchful waiting and adjuvant imatinib therapy may be appropriate for these patients.[1,2]
In general, gastric GISTs may be removed by laparoscopic wedge resection, when technically feasible. GISTs rarely involve the locoregional lymph nodes. Thus, extensive lymph node dissection is not indicated unless there is clinically apparent nodal involvement. These tumors may have fragile pseudocapsules, so care must be taken to avoid rupturing the pseudocapsule during surgery, which could result in peritoneal dissemination.
Postoperative adjuvant TKI therapy
Imatinib
Results from three phase III studies support the use of postoperative adjuvant imatinib for patients with completely resected localized GISTs who have a high risk of recurrence based on tumor size, tumor location, mitotic index, and presence of tumor rupture.[3,4,5,6,7,8,9,10]
Evidence (phase III studies of postoperative imatinib):
- ACOSOG Z9001 (NCT00041197) was a phase III, double-blind, placebo-controlled trial of 713 patients with fully resected KIT-mutant GISTs measuring at least 3 cm. Patients were randomly assigned to receive either imatinib 400 mg daily (n = 359) or placebo (n = 354) for 1 year after surgical resection.[4]
- After a median follow-up of 19.7 months, disease recurrence or death occurred in 30 patients (8.4%) in the imatinib arm and 70 patients (19.8%) in the placebo arm.
- The 1-year recurrence-free survival (RFS) rate was 98% in patients who received imatinib (95% confidence interval [CI], 96%–100%) and 83% (95% CI, 78%–88%) in patients who received placebo (hazard ratio [HR], 0.35; 95% CI, 0.22–0.53; P < .0001).[4][Level of evidence B1] No difference was noted in overall survival (OS) (HR, 0.66; 95% CI, 0.22–2.03; P = .4714).
- Dose-reduction or interruption because of adverse events occurred in 14.5% of patients in the imatinib arm and 2.8% of patients in the placebo arm. Grade 3 or 4 events occurred in 30.9% of patients in the imatinib arm and 18.3% of patients in the placebo arm.
- EORTC-62024 (NCT00103168) was a phase III open-label trial of 908 patients with fully resected (R0 or R1 margin) KIT-mutant GISTs at intermediate or high risk of recurrence. Patients were randomly assigned to receive either imatinib 400 mg daily (n = 454) or observation (n = 454) for 2 years.[10]
- At a median follow-up of 4.7 years, RFS rates were improved for patients who received imatinib compared with patients who underwent observation at 3 years (84% vs. 66%) and 5 years (69% vs. 63%) (log-rank P < .001).[10][Level of evidence B1] The 5-year OS rate did not differ between the imatinib and observation arms (91.8% vs. 92.7%).
- The 5-year imatinib failure-free survival rate (day of randomization to the start of a new systemic treatment or death) was 87% in the imatinib arm and 84% in the observation arm (HR, 0.79; 98.5% CI, 0.50–1.25; P = .21).[10][Level of evidence B1]
- A final analysis at a median follow-up of 9.1 years showed RFS rates of 70% and 63% at 5 and 10 years, respectively, for patients in the imatinib arm, and rates of 63% and 61% at 5 and 10 years, respectively, for patients in the observation arm (HR, 0.71; 95% CI, 0.57–0.89; P = .002). There was no difference in OS between patients who received imatinib and patients who underwent observation (93% vs. 92% at 5 years, 80% vs. 78% at 10 years; HR, 0.88; 95% CI, 0.65–1.21; P = .43).[9][Levels of evidence B1 and A1]
- SSG XVIII (NCT00116935) was a phase III open-label trial of 400 patients with fully resected, high-risk GISTs. Patients were randomly assigned to receive imatinib 400 mg daily for either 1 year (n = 200) or 3 years (n = 200) after resection.[5]
- After a median follow-up of 54 months, the RFS rate was 65.6% in the 3-year arm and 47.9% in the 1-year arm (HR, 0.46; 95% CI, 0.32–0.65; P < .001).[5][Level of evidence B1]
- The 5-year OS rate was 92% in the 3-year arm and 81.7% in the 1-year arm (HR, 0.45; 95% CI, 0.22–0.89; P = .02).[5]
- Although generally well-tolerated in both groups, grade 3 or 4 events occurred in 32.8% of patients in 3-year arm and 20.1% of patients in the 1-year arm. Treatment discontinuation occurred in 25.8% of patients in 3-year arm and 12.6% of patients in the 1-year arm.
- A post-hoc exploratory analysis suggested that patients with KIT exon 11–mutated GISTs derived the most benefit from a longer duration of imatinib (5-year RFS, 71.0% vs. 41.3%; P < .001).[6]
The recommended length of adjuvant treatment remains unknown. However, based on the SSG XVIII study results, at least 3 years of therapy is generally used in practice. It is important to note that evidence suggests that, instead of being cytotoxic, imatinib may suppress GIST growth. Therefore, recurrence may be delayed by the suppression of undetectable metastatic disease.[5,11,12,13] For example, the rate of recurrence increased within 6 to 12 months of discontinuing adjuvant imatinib in both the 1-year and 3-year arms in the SSG XVIII trial.[5] This concept has led to higher-risk patients being given imatinib indefinitely, although there is no direct trial evidence to support that.
Most patients initiate imatinib therapy at a dosage of 400 mg per day. Molecular genotyping of patients with GISTs is recommended as it can impact the use of adjuvant imatinib, as well as the optimal dose. Patients whose tumor harbors a KIT exon 9 mutation may benefit from higher-dose imatinib (800 mg per day) based on data in the metastatic setting.[14] Patients with KIT/PDGFRA wild-type GISTs (i.e., SDH-deficient and neurofibromatosis type 1 [NF1]-related GISTs) or PDGFRA D842V-mutant GISTs are unlikely to benefit from adjuvant imatinib therapy.[5]
Although not fully conclusive, there is some phase II evidence to support continuing adjuvant imatinib therapy for 5 years or more.
Evidence (phase II studies of postoperative imatinib):
- PERSIST-5 (NCT00867113) was a single-arm phase II trial of 91 patients with fully resected, high-risk GISTs. Patients received imatinib (400 mg daily) for up to 5 years.[15][Level of evidence C1]
- The median treatment duration was 55.1 months, but with a large range (0.5–60.6 months). Only 46 patients (51%) completed all 5 years of therapy. Thus, 49% of patients stopped treatment early because of patient choice (21%), adverse events (16%), or other reasons (12%).
- At a median follow-up of 19.6 months, the estimated 5-year RFS rate was 90% (95% CI, 80%–95%). The OS rate was 95% (95% CI, 86%–99%). Seven patients (7.6%) had a recurrence, 6 of which occurred after treatment discontinuation.
- A small, single-institution, retrospective analysis included 234 patients with R0-resected GISTs at moderate to high risk of recurrence. The study evaluated the effect of differing durations of postoperative imatinib on 5-year RFS and OS rates.[13][Level of evidence C1]
- At a median follow-up of 54 months, the 5-year RFS rate across all groups was 76.2%. The OS rate across all groups was 83.4%.
- In high-risk patients, longer durations of imatinib therapy were associated with higher RFS rates (36.5% in the 1-year group, 68.7% in the 1–3-years group, 71.2% in the 3–5-years group, and 90.8% in the >5-years group; P < .001). Longer imatinib therapy duration was also associated with higher OS rates (36.7% in the 1-year group, 76.6% in the 1–3-years group, 84.0% in the 3–5-years group, and 97.4% in the >5-years group; P < .001).
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
-
Demetri GD, Benjamin RS, Blanke CD, et al.: NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5 (Suppl 2): S1-29; quiz S30, 2007.
-
Otani Y, Furukawa T, Yoshida M, et al.: Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery 139 (4): 484-92, 2006.
-
DeMatteo RP, Owzar K, Antonescu CR, et al.: Efficacy of adjuvant imatinib mesylate following complete resection of localized, primary gastrointestinal stromal tumor (GIST) at high risk of recurrence: the U.S. Intergroup phase II trial ACOSOG Z9000. [Abstract] American Society of Clinical Oncology 2008 Gastrointestinal Cancers Symposium, 25-27 January 2008, Orlando, FL. A-8, 2008.
-
Dematteo RP, Ballman KV, Antonescu CR, et al.: Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373 (9669): 1097-104, 2009.
-
Joensuu H, Eriksson M, Sundby Hall K, et al.: One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 307 (12): 1265-72, 2012.
-
Joensuu H, Wardelmann E, Sihto H, et al.: Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial. JAMA Oncol 3 (5): 602-609, 2017.
-
Raut CP, Espat NJ, Maki RG, et al.: Extended treatment with adjuvant imatinib (IM) for patients (pts) with high-risk primary gastrointestinal stromal tumor (GIST): The PERSIST-5 study. [Abstract] J Clin Oncol 35 (Suppl 15): A-11009, 2017. Also available online. Last accessed June 5, 2019.
-
DeMatteo RP, Ballman KV, Antonescu CR, et al.: Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 258 (3): 422-9, 2013.
-
Casali PG, Le Cesne A, Velasco AP, et al.: Final analysis of the randomized trial on imatinib as an adjuvant in localized gastrointestinal stromal tumors (GIST) from the EORTC Soft Tissue and Bone Sarcoma Group (STBSG), the Australasian Gastro-Intestinal Trials Group (AGITG), UNICANCER, French Sarcoma Group (FSG), Italian Sarcoma Group (ISG), and Spanish Group for Research on Sarcomas (GEIS)☆. Ann Oncol 32 (4): 533-541, 2021.
-
Casali PG, Le Cesne A, Poveda Velasco A, et al.: Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 33 (36): 4276-83, 2015.
-
Joensuu H, Eriksson M, Sundby Hall K, et al.: Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 34 (3): 244-50, 2016.
-
Blanke CD, DeMatteo RP: Duration of Adjuvant Therapy for Patients With Gastrointestinal Stromal Tumors: Where Is Goldilocks When We Need Her? JAMA Oncol 2 (6): 721-2, 2016.
-
Lin JX, Chen QF, Zheng CH, et al.: Is 3-years duration of adjuvant imatinib mesylate treatment sufficient for patients with high-risk gastrointestinal stromal tumor? A study based on long-term follow-up. J Cancer Res Clin Oncol 143 (4): 727-734, 2017.
-
Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST): Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 28 (7): 1247-53, 2010.
-
Raut CP, Espat NJ, Maki RG, et al.: Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients With Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol 4 (12): e184060, 2018.
Treatment of Unresectable Primary GISTs
Treatment Options for Unresectable Primary GISTs
Treatment options for unresectable primary gastrointestinal stromal tumors (GISTs) include the following:
- Neoadjuvant tyrosine kinase inhibitor (TKI) therapy.
Neoadjuvant TKI therapy
Imatinib
Until surgical therapy is feasible, which can take as long as 6 to 12 months, therapy with neoadjuvant imatinib may be used for patients with very large primary GISTs or poorly positioned small GISTs (considered unresectable without the risk of significant morbidity or functional deficit, such as needing a formal gastrectomy, pancreatectomy, or other major organ resection).[1,2] Neoadjuvant imatinib therapy in patients with GISTs is supported by the early results of two phase II studies in the United States [3] and Asia [4], as well as several case series and small retrospective reports.[2,5,6,7,8,9,10] Neoadjuvant imatinib may be particularly beneficial in rectal GISTs given the large bulky nature of the disease and the extensive surgery required for complete resection.[11,12]
Evidence (phase II studies of neoadjuvant imatinib):
- RTOG-0132/ACRIN-6665 (NCT00028002) was a phase II single-arm study of 52 patients with primary GISTs (n = 30) or operable metastatic GISTs (n = 22). Patients received preoperative imatinib (600 mg daily) for 8 to 12 weeks followed by postoperative imatinib for at least 2 years.[3][Level of evidence C3]
- Among patients with primary GISTs, 83% had stable disease and 7% had a partial response (7%). Among patients with metastatic GISTs, 91% had stable disease, 4.5% had a partial response, and 4.5% had disease progression.
- At a median follow-up of 36 months, the 2-year PFS rate was 83% in patients with primary GISTs and 77% in patients with metastatic GISTs. The OS rate was 93% in patients with primary GISTs and 91% in patients with metastatic GISTs.
- Seventy-seven percent of patients with primary GISTs and 58% of patients with metastatic GISTs went on to have R0 resections. Five patients (10%) had unresectable disease.
- Imatinib was generally well tolerated, although 35% of patients had grade 3 to 5 adverse events. The median preoperative duration of imatinib was 65 days, and the median time of imatinib discontinuation before surgery was 2 days.
- A phase II single-arm study conducted in Asia included 53 evaluable patients with gastric GISTs larger than 10 cm. Patients received preoperative imatinib (400 mg daily) for 6 to 9 months followed by at least 1 year of postoperative imatinib.[4][Level of evidence C3]
- Forty-six patients (87%) received at least 6 months of preoperative imatinib and 50 patients ultimately underwent gastrectomy. The median duration of preoperative imatinib was 26 weeks. The most common grade 3 to 4 adverse events were neutropenia and rash.
- The objective response rate was 62%, and the maximal reduction occurred most commonly at 24 weeks (63% of patients). The R0 resection rate was 91% overall, and at least one-half of the stomach was preserved in 79% of patients.
- At a median follow-up of 32 months, the 2-year PFS rate was 89%, and the OS rate was 98%.
If a preoperative TKI is planned, a biopsy for diagnosis confirmation and, potentially, molecular profiling should be considered. Mutational analysis may help to exclude nonsensitive mutations before starting imatinib cytoreduction therapy. Biopsy and molecular profiling may also determine whether a tumor harbors a KIT exon 9 mutation, which may require an increase in initial imatinib dosing.[1,13] Neoadjuvant imatinib is not used for patients with GISTs harboring a PDGFRA D842V mutation. Some guidelines, such as those from the European Society of Medical Oncology, recommend considering neoadjuvant avapritinib.[14] However, avapritinib has not been tested or validated in the neoadjuvant setting. In addition, patients with KIT/PDGFRA wild-type GISTs (i.e., SDH-deficient or neurofibromatosis type 1 [NF1]-related GISTs) would not benefit from neoadjuvant therapy and should proceed directly to surgery, if feasible.
If indicated, neoadjuvant imatinib is initiated at 400 mg per day in most patients. Patients with KIT exon 9–mutated GISTs may be offered a higher dose (800 mg per day) based on data from the advanced setting.[15] Follow-up imaging, with either computed tomography (CT) or positron emission tomography (PET)-CT, is performed at close intervals. PET-CT can be particularly helpful in assessing initial early response if baseline molecular profiling was not done before neoadjuvant therapy.[16] The optimal duration of neoadjuvant treatment is unknown and should be individualized based on multidisciplinary discussion. Neoadjuvant TKI therapy precludes the ability to risk stratify after surgical resection. Therefore, patients should continue imatinib after surgery for at least 3 total years.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
-
Demetri GD, Benjamin RS, Blanke CD, et al.: NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5 (Suppl 2): S1-29; quiz S30, 2007.
-
Bonvalot S, Eldweny H, Péchoux CL, et al.: Impact of surgery on advanced gastrointestinal stromal tumors (GIST) in the imatinib era. Ann Surg Oncol 13 (12): 1596-603, 2006.
-
Eisenberg BL, Harris J, Blanke CD, et al.: Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 99 (1): 42-7, 2009.
-
Kurokawa Y, Yang HK, Cho H, et al.: Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer 117 (1): 25-32, 2017.
-
Andtbacka RH, Ng CS, Scaife CL, et al.: Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol 14 (1): 14-24, 2007.
-
Katz D, Segal A, Alberton Y, et al.: Neoadjuvant imatinib for unresectable gastrointestinal stromal tumor. Anticancer Drugs 15 (6): 599-602, 2004.
-
Raut CP, Posner M, Desai J, et al.: Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 24 (15): 2325-31, 2006.
-
Scaife CL, Hunt KK, Patel SR, et al.: Is there a role for surgery in patients with "unresectable" cKIT+ gastrointestinal stromal tumors treated with imatinib mesylate? Am J Surg 186 (6): 665-9, 2003.
-
Machlenkin S, Pinsk I, Tulchinsky H, et al.: The effect of neoadjuvant Imatinib therapy on outcome and survival after rectal gastrointestinal stromal tumour. Colorectal Dis 13 (10): 1110-5, 2011.
-
Rutkowski P, Gronchi A, Hohenberger P, et al.: Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 20 (9): 2937-43, 2013.
-
Cavnar MJ, Wang L, Balachandran VP, et al.: Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann Surg Oncol 24 (13): 3972-3980, 2017.
-
Tielen R, Verhoef C, van Coevorden F, et al.: Surgical management of rectal gastrointestinal stromal tumors. J Surg Oncol 107 (4): 320-3, 2013.
-
Debiec-Rychter M, Sciot R, Le Cesne A, et al.: KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42 (8): 1093-103, 2006.
-
Casali PG, Blay JY, Abecassis N, et al.: Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 33 (1): 20-33, 2022.
-
Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST): Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 28 (7): 1247-53, 2010.
-
Van den Abbeele AD, Gatsonis C, de Vries DJ, et al.: ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression. J Nucl Med 53 (4): 567-74, 2012.
Treatment of Metastatic or Recurrent GISTs
Treatment Options for Metastatic or Recurrent GISTs
Treatment options for metastatic or recurrent gastrointestinal stromal tumors (GISTs) include the following:
- Initial tyrosine kinase inhibitor (TKI) therapy.
- Initial TKI therapy for PDGFRA D842-mutant GISTs.
- TKI therapy for imatinib-resistant GISTs.
- Additional TKI therapy options.
- Ripretinib.
- Nilotinib.
- Sorafenib.
- Pazopanib.
- TKI therapy for KIT/PDGFRA wild-type GISTs.
- Surgery.
- Clinical trials.
The primary treatment of patients with metastatic or recurrent GISTs involves medical therapy with a TKI. In select cases, surgical therapy may be added. Patients with metastatic or recurrent tumors that do not respond to these measures may be candidates for clinical trials.
Initial TKI therapy
Imatinib
Therapy with imatinib is the standard first-line treatment for most patients with metastatic or recurrent disease. The initial dose is 400 mg daily, except for patients with tumors containing KIT exon 9 mutations, who may receive 800 mg daily.[1] The only exception is for patients with GISTs characterized by the PDGFRA D842V mutation. In this subtype, avapritinib is used as first-line therapy given high clinical benefit and imatinib resistance.[2] Most patients can initiate imatinib empirically while awaiting confirmation of their tumor's molecular profile. That profile may necessitate an imatinib dosing change (i.e., KIT exon 9), a change to avapritinib (i.e., PDGFRA D842V mutation), or indicate likelihood for TKI resistance (i.e., SDH-deficient or neurofibromatosis type 1 [NF1]-related GISTs).
All patients receiving TKI therapy are closely monitored for tumor response and side effects, which may require dose reductions, interruptions, or cessation of TKI therapy in cases of persistent, excessive toxicity. In addition, dose modification of the TKI or substitution with medications that do not affect cytochrome P450 isoenzyme 3A4 (CYP450 3A4) levels may be necessary for patients taking drugs that affect CYP450 3A4 levels.[3]
Response is evaluated with computed tomography (CT), magnetic resonance imaging (MRI), or fluorine F 18-fludeoxyglucose positron emission tomography (18F-FDG PET).[3,4,5,6,7] Treatment is usually continued indefinitely in the absence of disease progression or unacceptable toxicity, with a median time to progression of 24 to 40 months and median survival approaching 45 to 60 months.[3,8,9,10,11,12,13,14] A cohort of patients from early imatinib trials have continued on therapy with long-term survival. In a multivariable analysis, age younger than 60 years, performance status 0, smaller size of the largest lesion, and exon 11 KIT mutation were significant prognostic factors for the probability of surviving beyond 10 years.[10] A similar finding for exon 11 was seen in a phase II study.[9]
Evidence (imatinib therapy):
- A phase III trial included 746 patients with advanced unresectable or metastatic GISTs. Patients were randomly assigned to receive either higher-dose treatment with 800 mg imatinib daily or 400 mg imatinib daily as primary systemic therapy.
- No statistically significant differences in objective response rates, progression-free survival (PFS), or overall survival (OS) were observed between patients who received the 800 mg dose and patients who received the 400 mg dose.[15][Levels of evidence A1; B1; and B3]
- Among patients who progressed at 400 mg daily and crossed over to 800 mg daily, approximately one-third were able to achieve an objective response or disease stabilization.
- Similar findings were seen in a European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EORTC-ISG-AGITG) study of 946 patients with GISTs who were randomly assigned to receive 400 mg or 800 mg of imatinib, with crossover permitted at progression.[16]
- It is now recognized that specific kinase mutations in KIT and PDGFRA impact sensitivity/response to imatinib (e.g., exon 11 is imatinib sensitive and exon 9 is imatinib resistant).[17,18,19,20,21] In addition, it has been demonstrated in meta-analyses of both trials mentioned above that patients with KIT exon 9 mutations have significant benefit with higher-dose imatinib.[22]
In the event of tumor progression in patients without KIT exon 9 mutations on lower dose imatinib (i.e., 400 mg daily), the imatinib dosage may be increased to 800 mg daily (in split doses). Alternatively, in the management of imatinib resistance, the patient may be switched directly to sunitinib.[23]
The most common toxicities associated with imatinib therapy, all of which may improve with prolonged treatment, include the following:[6,11,24,25,26]
- Fluid retention (especially periorbital edema or peripheral edema; occasionally pleural effusion or ascites).
- Diarrhea.
- Nausea (may be diminished if taken with food).
- Fatigue.
- Muscle cramps.
- Abdominal pain.
- Rash.
- Mild (macrocytic) anemia.
- Hypophosphatemia.
There are rare reports of heart failure related to imatinib use,[27] primarily in patients with preexisting heart disease. No excess cardiac toxicity was noted in either of the phase III trials of imatinib mentioned above for patients with advanced GISTs.[15,16] However, it is best to inform patients of this risk before starting imatinib and monitor clinically for signs of heart failure or left ventricular dysfunction.
Initial TKI therapy forPDGFRAD842V-mutant GISTs
Avapritinib
Patients with GISTs that harbor a PDGFRA exon 18 D842V mutation should initially be given avapritinib. However, for patients whose GISTs are asymptomatic or indolent, a period of observation is reasonable to avoid treatment toxicities.
Evidence (avapritinib in patients with a PDGFRA D842V mutation):
- NAVIGATOR (NCT02508532) was a phase I, single-arm, open-label trial of 56 patients with a PDGFRA D842V mutation. Patients received avapritinib at a daily dose of either 300 mg or 400 mg.[2][Level of evidence C3]
- An overall response was seen in 49 of 56 patients (88%) (95% confidence interval [CI], 76%–95%), with five patients (9%) achieving a complete response.
- The 1-year PFS rate was 81% (95% CI, 67%–94%) and the 1-year duration of response was 70% (95% CI, 54%–87%).
- At median follow-up of 15.9 months, the estimated 1-year OS rate was 91%, and the estimated 2-year OS rate was 81%.
- The high overall response rate results of the NAVIGATOR trial led the U.S. Food and Drug Administration (FDA) to approve avapritinib for patients with GISTs with a PDGFRA exon 18 mutation, including D842V mutations.
Evidence (avapritinib in patients who did not respond to imatinib and at least one additional TKI):
- VOYAGER (NCT03465722) was a phase III open-label trial of 476 patients with advanced GISTs that did not respond to imatinib and at least one additional TKI. Patients were randomly assigned to receive either avapritinib 300 mg daily (n = 240) or regorafenib 160 mg daily (3-weeks-on/1-week-off regimen) (n = 236), with crossover allowed from regorafenib to avapritinib.[28]
- The median PFS was 4.2 months in the avapritinib arm and 5.6 months in the regorafenib arm (hazard ratio [HR], 1.25; 95% CI, 0.99–1.57; P = .055). Among patients without a PDGFRA D842V mutation, the median PFS was 3.9 months in the avapritinib arm and 5.6 months in the regorafenib arm (HR, 1.34; 95% CI, 1.06–1.69; P = .012).[28][Level of evidence B1]
- OS data were immature at the time of the report with no interval differences noted between the study arms.
- Overall and grade 3 or higher treatment-related adverse events did not differ between groups. However, cognitive effects occurred more often in patients who received avapritinib (25.9%) than in patients who received regorafenib (3.8%).
- Because avapritinib did not improve PFS or OS compared with regorafenib in the treated population, it is not indicated until patients have failed multiple previous TKI therapies (outside of its specific mutational indication above).
If indicated, avapritinib is given at 300 mg daily. Avapritinib is teratogenic, and thus, warrants effective contraception during and up to 6 weeks after the final dose.[29] The 300 mg dose was generally well-tolerated in the phase I NAVIGATOR study, with grade 3 to 4 toxicities including anemia, hyperbilirubinemia, fatigue, abdominal pain, diarrhea, peripheral edema, pleural effusion, and cognitive impairment.[2]
Cognitive effects must be closely monitored, with treatment changes (reductions, modifications, discontinuation) made promptly. Based on a post-hoc analysis of patients receiving 300 mg daily, grade 1 to 2 cognitive effects were seen in 37% of patients and 52% of patients older than 65 years. These effects included cognitive impairment, mood changes, sleep disorder, dizziness, hallucinations, and intracranial hemorrhage. These cognitive effects generally improved once treatment changes were made.[29]
Of note, for patients with GISTs who do not harbor a PDGFRA D842V mutation, avapritinib should not be used until imatinib and at least two additional agents (sunitinib and regorafenib) are tried. The open-label phase III VOYAGER trial demonstrated that regorafenib improved PFS more than avapritinib in patients without a PDGFRA D842V mutation.[28]
TKI therapy for imatinib-resistant GISTs
Sunitinib
In the case of tumor progression (or intolerance to imatinib), data support second-line therapy with either imatinib dose escalation to 800 mg per day (as described above) or sunitinib.[16,21] Sunitinib is given at a dose of 50 mg daily in a 4-weeks-on/2-weeks-off regimen or a daily dose of 37.5 mg.[30] As with imatinib, the response to sunitinib is evaluated with CT, MRI, or 18F-FDG PET, and treatment is usually continued indefinitely in the absence of disease progression or unacceptable toxicity.[3,4,30,31,32,33,34,35,36]
Evidence (sunitinib):
- An international phase III trial of 312 patients with imatinib-resistant GISTs randomly assigned patients to receive sunitinib or placebo.[30]
- On the basis of radiological assessment, the median time to tumor progression was more than four times as long with sunitinib (27.3 weeks; 95% CI, 16.0–32.1) than with placebo treatment (6.4 weeks; 95% CI, 4.4–10.0) (HR, 0.33; 95% CI, 0.23–0.47; P < .0001).[30][Level of evidence A1]
- OS was similarly better for sunitinib-treated patients (HRdeath, 0.49; 95% CI, 0.29–0.83).[30][Level of evidence A1]
The response to sunitinib is also influenced by the molecular profile of the GIST. Based on a phase I/II study of 97 patients, the highest clinical benefit rate, PFS benefit, and OS benefit were seen in patients with KIT exon 9 mutations, compared with patients with KIT/PDGFRA wild-type or KIT exon 11 mutations.[33]
Common side effects associated with sunitinib include the following:[30,37]
- Fatigue.
- Nausea and vomiting.
- Anemia.
- Neutropenia.
- Diarrhea.
- Abdominal pain.
- Mucositis.
- Anorexia.
- Skin or hair discoloration.
- Proteinuria.
- Hypothyroidism (thyroid function monitoring is generally recommended).
- Hypertension.
- Potential for delayed wound healing (may require holding 3–4 days prior to surgery).
Less frequent toxicities include bleeding, fever, and hand-foot syndrome.[30] Therapy with sunitinib may be cardiotoxic. In a retrospective phase I/II study evaluating the efficacy of sunitinib in patients with imatinib-resistant metastatic GISTs, 8% of patients who received repeated cycles of sunitinib experienced congestive heart failure, while 47% of patients developed hypertension (>150 per 100 mm Hg). Reductions in left ventricular ejection fraction were seen in at least 10% to 28% of patients.[38]
Regorafenib
The FDA has approved regorafenib for the treatment of GISTs that are refractory to first-line therapy. Regorafenib is a multikinase inhibitor with activity against KIT, PDGFRA, and VEGFR, among others. Regorafenib has demonstrated anti-GIST activity in phase II and phase III studies.[39,40]
Evidence (regorafenib):
- The phase III double-blind GRID trial (NCT01271712) included 199 patients with advanced GISTs who did not respond to previous imatinib and sunitinib therapy. Patients were randomly assigned in a 2:1 ratio to receive either 160 mg daily of regorafenib (3-weeks-on/1-week-off regimen) (n = 133) or placebo (n = 66). Crossover to open-label regorafenib was allowed for patients who had disease progression on the placebo arm.[40]
- After a median treatment duration of 23 weeks for regorafenib and 7 weeks for placebo, the median PFS was longer in patients who received regorafenib (4.8 months) compared with patients who received placebo (0.9 months) (HR, 0.27; 95% CI, 0.19–0.39; P < .0001).[40][Level of evidence B1] The OS did not differ between arms (HR, 0.77; 0.4–1.41; P = .199). However, 56 patients in the placebo arm (85%) did cross over to receive regorafenib at the time of disease progression.
- Adverse events were more common in the regorafenib arm (98.5%) compared with the placebo arm (68.2%). The most common grade 3 or greater events were hypertension (23.5%), hand-foot syndrome (19.7%), and diarrhea (5.3%).
Additional TKI therapy options
Ripretinib
Ripretinib is indicated for patients with advanced GISTs who have disease progression on (or are intolerant to) three or more TKIs, including imatinib. It works as a switch control inhibitor with multiple targets, including KIT exons 9, 11, 13, 14, 18, and it stabilizes the KIT molecule in its active form.
Based on the toxicity profile of ripretinib, a baseline echocardiogram or multigated acquisition (MUGA) scan should be obtained, and blood pressure and clinical signs of heart failure should be serially monitored. Dermatologic exams are warranted, given the association with the development of cutaneous cancers and hand-foot syndrome. Ripretinib is teratogenic and warrants concomitant effective contraception. It should also not be given perioperatively (1 week before or 2 weeks after surgery) because of the risk of delayed wound healing.[41,42]
Evidence (ripretinib):
- INVICTUS (NCT03353753) was a phase III, double-blind, placebo-controlled trial of 129 patients with advanced GISTs who had not responded to previous imatinib, sunitinib, and regorafenib therapy. Patients were randomly assigned in a 2:1 ratio to receive either ripretinib 150 mg daily (n = 85) or placebo (n = 44), with an opportunity to cross over at the time of disease progression.[41]
- After a median follow-up of 6.3 months for the ripretinib arm and 1.6 months for the placebo arm, the median OS was longer for patients who received ripretinib (15.1 months), compared with patients who received placebo (6.6 months) (HR, 0.36; 95% CI, 0.21–0.62).[41][Level of evidence A1]
- The median PFS was longer for patients who received ripretinib (6.3 months) than patients for who received placebo (1.0 month) (HR, 0.15; 95% CI, 0.09–0.25; P < .0001). The PFS rate at 6 months was 51% in the ripretinib arm (95% CI, 39.4%–61.4%) and 3.2% in the placebo arm (95% CI, 0.2%–13.8%).[41]
- Twenty-nine patients (66%) in the placebo arm crossed over to the ripretinib arm.
- Ripretinib was well tolerated. The most common grade 3 or 4 adverse events were lipase increase (5%), hypertension (4%), fatigue (2%), and hypophosphatemia (2%).
- INTRIGUE (NCT03673501) was an open-label phase III trial of 453 patients with advanced GISTs who did not respond to imatinib therapy. Patients were randomly assigned to receive either ripretinib 150 mg daily (n = 226) or sunitinib 50 mg (4-weeks-on/2-weeks-off regimen; n = 227).[43]
- The median PFS did not differ between the ripretinib and sunitinib arms (8.0 months vs. 8.3 months; HR, 1.05; 95% CI, 0.82–1.33; P = .72). In addition, there was no difference in the median PFS in the KIT exon 11 cohort (8.3 months vs. 7.0 months; HR, 0.88; 95% CI, 0.66–1.16; P = .36).[43][Level of evidence B1]
- OS data were immature at the time of report.
- Patients who received ripretinib had fewer treatment-emergent adverse events (41.3% vs. 65.6%; P < .0001) and were more likely to develop drug-related grade 3 or 4 hypertension (5.8% vs. 22.6%). Dose modifications, interruptions, or discontinuations were all less common with ripretinib.
- Ripretinib did not lead to a PFS or OS benefit when compared with sunitinib. Therefore, it is not indicated unless the patient's tumor does not respond to prior lines of TKI therapy.
Nilotinib
Nilotinib is a second-generation TKI with similar targets to imatinib. A phase III study of nilotinib versus best supportive care in imatinib- and sunitinib-resistant GISTs showed some PFS benefit based on local assessment but no PFS benefit based on central assessment. Post-hoc analysis did reveal a modest but significant median OS difference of 4 months.[44]
Sorafenib
Sorafenib is a multitarget kinase that is similar in structure and mechanism to regorafenib. A phase II trial of patients with imatinib- and sunitinib-resistant GISTs showed that sorafenib offered potential benefit, with a disease control rate of 68% and a median PFS of 5 months.[45]
Pazopanib
Pazopanib inhibits VEGF signaling and showed modest PFS benefit when compared to best supportive care in a small phase II trial of patients with imatinib- and sunitinib-resistant GISTs. However, pazopanib had a high rate of toxicity.[46]
TKI therapy forKIT/PDGFRAwild-type GISTs
Patients without KIT or PDGFRA mutations, such as SDH-deficient and NF1-related GISTs, do not benefit from initial TKI treatment with imatinib. However, these patients may have modest response to sunitinib and regorafenib.[47] These tumors tend to have a relatively indolent course, and optimal management of these patients remains unknown. Thus, patients should be encouraged to enroll in a clinical trial, if available.
Surgery
Surgery may be added to medical therapy for selected patients with GISTs in an effort to delay or prevent recurrence, although the benefit of this therapeutic approach in patients with metastatic GISTs has yet to be proven in a randomized clinical trial.
Evidence (surgery):
- A retrospective study involving 69 consecutive patients who underwent surgery for unresectable primary or metastatic GISTs while receiving kinase inhibitors reported the following:[48][Levels of evidence C2 and C1]
- Patients with stable disease or limited progression were found to have prolonged survival after debulking procedures. In this group of patients with GISTs, no evidence of disease was found after surgery in 78% of patients with stable disease, 25% of patients with limited progression, and 7% of patients with generalized progression.
- The 12-month PFS rate was 80% for patients with stable disease, 33% for patients with limited progression, and 0% for patients with generalized progression.
- The 12-month OS rate was 95% for patients with stable disease, 86% for patients with limited progression, and 0% for patients with generalized progression.
- The authors of this study concluded that surgery for patients with generalized progression should be limited to a palliative role.
Overall, the indications for surgery in the management of metastatic or recurrent GISTs include the following:[3]
- Stable disease (i.e., disease that is stable or shrinking on TKI therapy when gross resection is possible).
- Limited disease progression (i.e., isolated tumor deposits that are progressing on TKI therapy after initial response [indicating delayed drug resistance], while other sites of disease remain stable).
- Oncological emergencies including hemorrhage, perforation, obstruction, or abscess.
Stable disease and limited disease progression identify subsets of patients with advanced disease that are selected for relative disease stability. Therefore, the favorable outcomes that have been noted in case series may be principally the result of selection bias rather than true benefit from surgery.
The median time to the development of secondary resistance to imatinib has been about 2 years.[12] Therefore, it is suggested that surgery for metastatic or recurrent disease in patients receiving imatinib/sunitinib be performed before 2 years. Most experts would recommend considering surgery after 6 to 12 months of disease stability with TKI therapy.[3] Drug therapy may be continued after surgery.
Clinical trials
Patients who have generalized disease progression while receiving standard therapies, or with certain molecular subtypes (i.e., SDH-deficient or NF1-related GISTs) may benefit from enrolling in clinical trials. These patients should be referred to specialized multidisciplinary research centers.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
-
Debiec-Rychter M, Sciot R, Le Cesne A, et al.: KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42 (8): 1093-103, 2006.
-
Heinrich MC, Jones RL, von Mehren M, et al.: Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol 21 (7): 935-946, 2020.
-
Demetri GD, Benjamin RS, Blanke CD, et al.: NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5 (Suppl 2): S1-29; quiz S30, 2007.
-
Casali PG, Dei Tos AP, Gronchi A: Gastrointestinal stromal tumor. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, et al., eds.: DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. 11th ed. Wolters Kluwer, 2019, pp 895-906.
-
Blanke CD, von Mehren M, Joensuu H, et al.: Evaluation of the safety and efficacy of an oral molecularly-targeted therapy, STI157, in patients (pts) with unresectable or metastatic gastrointestinal stromal tumors (GISTs) expressing c-kit (CD117). [Abstract] Proceedings of the American Society of Clinical Oncology 20: A-1, 1a, 2001.
-
van Oosterom AT, Judson I, Verweij J, et al.: Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358 (9291): 1421-3, 2001.
-
Choi H, Charnsangavej C, Faria SC, et al.: Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25 (13): 1753-9, 2007.
-
Blay JY, Le Cesne A, Ray-Coquard I, et al.: Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 25 (9): 1107-13, 2007.
-
Blanke CD, Demetri GD, von Mehren M, et al.: Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26 (4): 620-5, 2008.
-
Casali PG, Zalcberg J, Le Cesne A, et al.: Ten-Year Progression-Free and Overall Survival in Patients With Unresectable or Metastatic GI Stromal Tumors: Long-Term Analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on Imatinib at Two Dose Levels. J Clin Oncol 35 (15): 1713-1720, 2017.
-
Demetri GD, von Mehren M, Blanke CD, et al.: Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347 (7): 472-80, 2002.
-
Verweij J, Casali PG, Zalcberg J, et al.: Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364 (9440): 1127-34, 2004.
-
Van Glabbeke M, Verweij J, Casali PG, et al.: Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 23 (24): 5795-804, 2005.
-
Rutkowski P, Nowecki ZI, Debiec-Rychter M, et al.: Predictive factors for long-term effects of imatinib therapy in patients with inoperable/metastatic CD117(+) gastrointestinal stromal tumors (GISTs). J Cancer Res Clin Oncol 133 (9): 589-97, 2007.
-
Blanke CD, Rankin C, Demetri GD, et al.: Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26 (4): 626-32, 2008.
-
Zalcberg JR, Verweij J, Casali PG, et al.: Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer 41 (12): 1751-7, 2005.
-
Heinrich MC, Corless CL, Demetri GD, et al.: Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21 (23): 4342-9, 2003.
-
Debiec-Rychter M, Dumez H, Judson I, et al.: Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 40 (5): 689-95, 2004.
-
Corless CL, Schroeder A, Griffith D, et al.: PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 23 (23): 5357-64, 2005.
-
Heinrich MC, Owzar K, Corless CL, et al.: Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol 26 (33): 5360-7, 2008.
-
Patrikidou A, Domont J, Chabaud S, et al.: Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer 52: 173-80, 2016.
-
van Glabbeke MM, Owzar K, Rankin C, et al.: Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors (GIST): a meta-analyis based on 1,640 patients (pts). [Abstract] J Clin Oncol 25 (Suppl 18): A-10004, 546s, 2007.
-
Rutkowski P, Nowecki Z, Nyckowski P, et al.: Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol 93 (4): 304-11, 2006.
-
Dagher R, Cohen M, Williams G, et al.: Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res 8 (10): 3034-8, 2002.
-
Benjamin RS, Rankin C, Fletcher C, et al.: Phase III dose-randomized study of imatinib mesylate (STI571) for GIST: Intergroup S0033 early results. [Abstract] Proceedings of the American Society of Clinical Oncology 22: A-3271, 2003.
-
Verweij J, van Oosterom A, Blay JY, et al.: Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 39 (14): 2006-11, 2003.
-
Kerkelä R, Grazette L, Yacobi R, et al.: Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 12 (8): 908-16, 2006.
-
Kang YK, George S, Jones RL, et al.: Avapritinib Versus Regorafenib in Locally Advanced Unresectable or Metastatic GI Stromal Tumor: A Randomized, Open-Label Phase III Study. J Clin Oncol 39 (28): 3128-3139, 2021.
-
Joseph CP, Abaricia SN, Angelis MA, et al.: Optimal Avapritinib Treatment Strategies for Patients with Metastatic or Unresectable Gastrointestinal Stromal Tumors. Oncologist 26 (4): e622-e631, 2021.
-
Demetri GD, van Oosterom AT, Garrett CR, et al.: Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368 (9544): 1329-38, 2006.
-
Prior JO, Montemurro M, Orcurto MV, et al.: Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol 27 (3): 439-45, 2009.
-
Demetri GD, Heinrich MC, Fletcher JA, et al.: Molecular target modulation, imaging, and clinical evaluation of gastrointestinal stromal tumor patients treated with sunitinib malate after imatinib failure. Clin Cancer Res 15 (18): 5902-9, 2009.
-
Heinrich MC, Maki RG, Corless CL, et al.: Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 26 (33): 5352-9, 2008.
-
Rutkowski P, Bylina E, Klimczak A, et al.: The outcome and predictive factors of sunitinib therapy in advanced gastrointestinal stromal tumors (GIST) after imatinib failure - one institution study. BMC Cancer 12: 107, 2012.
-
Demetri GD, Garrett CR, Schöffski P, et al.: Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res 18 (11): 3170-9, 2012.
-
Reichardt P, Kang YK, Rutkowski P, et al.: Clinical outcomes of patients with advanced gastrointestinal stromal tumors: safety and efficacy in a worldwide treatment-use trial of sunitinib. Cancer 121 (9): 1405-13, 2015.
-
Wolter P, Stefan C, Decallonne B, et al.: The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br J Cancer 99 (3): 448-54, 2008.
-
Chu TF, Rupnick MA, Kerkela R, et al.: Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370 (9604): 2011-9, 2007.
-
George S, Wang Q, Heinrich MC, et al.: Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol 30 (19): 2401-7, 2012.
-
Demetri GD, Reichardt P, Kang YK, et al.: Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381 (9863): 295-302, 2013.
-
Blay JY, Serrano C, Heinrich MC, et al.: Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 21 (7): 923-934, 2020.
-
Janku F, Abdul Razak AR, Chi P, et al.: Switch Control Inhibition of KIT and PDGFRA in Patients With Advanced Gastrointestinal Stromal Tumor: A Phase I Study of Ripretinib. J Clin Oncol 38 (28): 3294-3303, 2020.
-
Bauer S, Jones RL, Blay JY, et al.: Ripretinib Versus Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumor After Treatment With Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial. J Clin Oncol 40 (34): 3918-3928, 2022.
-
Reichardt P, Blay JY, Gelderblom H, et al.: Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol 23 (7): 1680-7, 2012.
-
Campbell NP, Wroblewski K, Maki RG, et al.: Final results of a University of Chicago phase II consortium trial of sorafenib (SOR) in patients (pts) with imatinib (IM)- and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST). [Abstract] J Clin Oncol 29 (4): A-4, 2011.
-
Mir O, Cropet C, Toulmonde M, et al.: Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol 17 (5): 632-41, 2016.
-
Boikos SA, Pappo AS, Killian JK, et al.: Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2 (7): 922-8, 2016.
-
Raut CP, Posner M, Desai J, et al.: Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 24 (15): 2325-31, 2006.
Treatment of Resistant or Refractory GISTs
Eventual development of resistance to imatinib, sunitinib, and regorafenib is nearly universal. There is no standard therapy when this occurs, and patients should consider investigational therapy, such as new oral tyrosine kinase inhibitors. If eligible, patients are encouraged to participate in clinical trials.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Key References for Treatment of GISTs
These references have been identified by members of the PDQ Adult Treatment Editorial Board as significant in the field of gastrointestinal stromal tumor (GIST) treatment. This list is provided to inform users of important studies that have helped shape the current understanding of and treatment options for GISTs. Listed after each reference are the sections within this summary where the reference is cited.
Preoperative Imatinib
8 to 12 weeks
- Eisenberg BL, Harris J, Blanke CD, et al.: Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 99 (1): 42-7, 2009. [PUBMED Abstract]
Cited in:
- Treatment of Unresectable Primary GISTs
6 to 9 months
- Kurokawa Y, Yang HK, Cho H, et al.: Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer 117 (1): 25-32, 2017. [PUBMED Abstract]
Cited in:
- Treatment of Unresectable Primary GISTs
Postoperative Imatinib
1 year of imatinib
- Dematteo RP, Ballman KV, Antonescu CR, et al.: Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373 (9669): 1097-104, 2009. [PUBMED Abstract]
Cited in:
- Treatment of Resectable Primary GISTs
2 years of imatinib
- Joensuu H, Eriksson M, Sundby Hall K, et al.: One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 307 (12): 1265-72, 2012. [PUBMED Abstract]
Cited in:
- Treatment of Resectable Primary GISTs
1 year versus 3 years of imatinib
- Casali PG, Le Cesne A, Poveda Velasco A, et al.: Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 33 (36): 4276-83, 2015. [PUBMED Abstract]
Cited in:
- Treatment of Resectable Primary GISTs
5 years of imatinib
- Raut CP, Espat NJ, Maki RG, et al.: Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients With Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol 4 (12): e184060, 2018. [PUBMED Abstract]
Cited in:
- Treatment of Resectable Primary GISTs
Varying duration of imatinib
- Lin JX, Chen QF, Zheng CH, et al.: Is 3-years duration of adjuvant imatinib mesylate treatment sufficient for patients with high-risk gastrointestinal stromal tumor? A study based on long-term follow-up. J Cancer Res Clin Oncol 143 (4): 727-734, 2017. [PUBMED Abstract]
Cited in:
- Treatment of Resectable Primary GISTs
Advanced GIST Tyrosine Kinase Inhibitors
Imatinib
- Demetri GD, von Mehren M, Blanke CD, et al.: Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347 (7): 472-80, 2002. [PUBMED Abstract]
Cited in:
- Treatment Option Overview for GISTs
- Treatment of Metastatic or Recurrent GISTs
- Blanke CD, Rankin C, Demetri GD, et al.: Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26 (4): 626-32, 2008. [PUBMED Abstract]
Cited in:
- Treatment of Metastatic or Recurrent GISTs
Avapritinib
- Heinrich MC, Jones RL, von Mehren M, et al.: Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol 21 (7): 935-946, 2020. [PUBMED Abstract]
Cited in:
- Treatment Option Overview for GISTs
- Treatment of Metastatic or Recurrent GISTs
Avapritinib versus regorafenib
- Kang YK, George S, Jones RL, et al.: Avapritinib Versus Regorafenib in Locally Advanced Unresectable or Metastatic GI Stromal Tumor: A Randomized, Open-Label Phase III Study. J Clin Oncol 39 (28): 3128-3139, 2021. [PUBMED Abstract]
Cited in:
- Treatment of Metastatic or Recurrent GISTs
Sunitinib
- Demetri GD, van Oosterom AT, Garrett CR, et al.: Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368 (9544): 1329-38, 2006. [PUBMED Abstract]
Cited in:
- Treatment of Metastatic or Recurrent GISTs
Regorafenib
- Demetri GD, Reichardt P, Kang YK, et al.: Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381 (9863): 295-302, 2013. [PUBMED Abstract]
Cited in:
- Treatment of Metastatic or Recurrent GISTs
Ripretinib
- Blay JY, Serrano C, Heinrich MC, et al.: Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 21 (7): 923-934, 2020. [PUBMED Abstract]
Cited in:
- Treatment of Metastatic or Recurrent GISTs
Ripretinib versus sunitinib
- Bauer S, Jones RL, Blay JY, et al.: Ripretinib Versus Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumor After Treatment With Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial. J Clin Oncol 40 (34): 3918-3928, 2022. [PUBMED Abstract]
Cited in:
- Treatment of Metastatic or Recurrent GISTs
Latest Updates to This Summary (05 / 11 / 2023)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
This summary was comprehensively reviewed, extensively revised, and reformatted.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of gastrointestinal stromal tumors. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Gastrointestinal Stromal Tumors Treatment are:
- Russell S. Berman, MD (New York University School of Medicine)
- Vinayak Venkataraman, MD
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Gastrointestinal Stromal Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/gist-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389157]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us.
Last Revised: 2023-05-11