General Information About Prostate Cancer
The median age at diagnosis of prostate cancer is 67 years.[1] Prostate cancer may be cured when localized, and it frequently responds to treatment when widespread. The rate of tumor growth varies from very slow to moderately rapid, and some patients may have prolonged survival even after the cancer has metastasized to distant sites, such as bone. The 5-year relative survival rate for men diagnosed in the United States from 2013 to 2019 with local or regional disease was greater than 99%, and the rate for distant disease was 34%; a 97% survival rate was observed for all stages combined.[2] The approach to treatment is influenced by age and coexisting medical problems. Side effects of various forms of treatment should be considered in selecting appropriate management.
Many patients—especially those with localized tumors—may die of other illnesses without ever having suffered disability from prostate cancer, even if managed conservatively without an attempt at curative therapy.[3,4] In part, these favorable outcomes are likely the result of widespread screening with the prostate-specific antigen (PSA) test, which can identify patients with asymptomatic tumors that have little or no lethal potential.[5] There is a large number of these clinically indolent tumors, estimated to range from 30% to 70% of men older than 60 years, based on autopsy series of men dying of causes unrelated to prostate cancer.[6,7]
Because diagnostic methods have changed over time, any analysis of survival after treatment of prostate cancer and comparison of the various treatment strategies is complicated by evidence of increasing diagnosis of nonlethal tumors. Nonrandomized comparisons of treatments may be confounded not only by patient selection factors but also by time trends.
For example, a population-based study in Sweden showed that, from 1960 to the late 1980s, before the use of PSA for screening purposes, long-term relative survival rates after the diagnosis of prostate cancer improved substantially as more sensitive methods of diagnosis were introduced. This occurred despite the use of watchful waiting or active surveillance or palliative hormonal treatment as the most common treatment strategies for localized prostate cancer during the entire era (<150 radical prostatectomies per year were performed in Sweden during the late 1980s). The investigators estimated that, if all prostate cancers diagnosed between 1960 and 1964 were of the lethal variety, then at least 33% of cancers diagnosed between 1980 and 1984 were of the nonlethal variety.[8][Level of evidence C1] With the advent of PSA screening as the most common method of detection in the United States, the ability to diagnose nonlethal prostate cancers has further increased.
Another issue complicating comparisons of outcomes among nonconcurrent series of patients is the possibility of changes in criteria for the histological diagnosis of prostate cancer.[9] This phenomenon creates a statistical artifact that can produce a false sense of therapeutic accomplishment and may also lead to more aggressive therapy.
Controversy exists about the value of screening, the most appropriate staging evaluation, and the optimal treatment of each stage of the disease.[10,11,12,13,14]
Incidence and Mortality
Estimated new cases and deaths from prostate cancer in the United States in 2024:[2][Cancer Stat Facts: Prostate Cancer]
- New cases: 299,010.
- Deaths: 35,250.
Anatomy
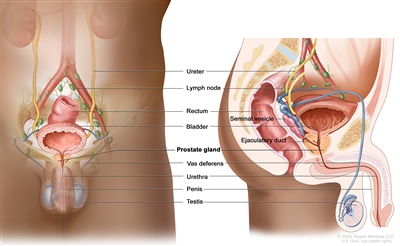
Figure 1. Anatomy of the male reproductive and urinary systems.
Screening
Screening for prostate cancer is controversial. In the United States, most prostate cancers are diagnosed because of screening, either with a PSA blood test or, less frequently, with a digital rectal examination. Randomized trials have yielded conflicting results.[15,16,17] Systematic literature reviews and meta-analyses have reported no clear evidence that screening for prostate cancer decreases the risk of death from prostate cancer, or that the benefits outweigh the harms of screening.[18,19]
For a detailed summary of evidence regarding the benefits and harms of screening for prostate cancer, see Prostate Cancer Screening.
Pathology
More than 95% of primary prostate cancers are adenocarcinomas. Prostate adenocarcinomas are frequently multifocal and heterogeneous in patterns of differentiation. Prostatic intraepithelial neoplasia (PIN) (noninvasive atypical epithelial cells within benign-appearing acini) is often present in association with prostatic adenocarcinoma. PIN is subdivided into low grade and high grade. The high-grade form may be a precursor of adenocarcinoma.[20]
Several rare tumors account for the rest of the cases. These include the following:
- Small-cell tumors.
- Intralobular acinar carcinomas.
- Ductal carcinomas.
- Clear cell carcinomas.
- Mucinous carcinomas.[21]
Gleason score
The histological grade of prostate adenocarcinomas is usually reported according to one of the variations of the Gleason scoring system, which provides a useful, albeit crude, adjunct to tumor staging in determining prognosis.[21] The Gleason score is calculated based on the dominant histological grades, from grade 1 (well differentiated) to grade 5 (very poorly differentiated). The classical score is derived by adding the two most prevalent pattern grades, yielding a score ranging from 2 to 10. Because there is some evidence that the least-differentiated component of the specimen may provide independent prognostic information, the score is often provided by its separate components (e.g., Gleason score 3 + 4 = 7; or 4 + 3 = 7).[22]
There is evidence that, over time, pathologists have tended to award higher Gleason scores to the same histological patterns, a phenomenon sometimes termed grade inflation.[23,24] This phenomenon complicates comparisons of outcomes in current versus historical patient series. For example, prostate biopsies from a population-based cohort of 1,858 men diagnosed with prostate cancer from 1990 through 1992 were re-read in 2002 to 2004.[23,24] The contemporary Gleason score readings were an average of 0.85 points higher (95% confidence interval, 0.79–0.91; P < .001) than the same slides read a decade earlier. As a result, Gleason-score standardized prostate cancer mortality rates for these men were artifactually improved from 2.08 to 1.50 deaths per 100-person years—a 28% decrease even though overall outcomes were unchanged.
Molecular markers
A number of tumor markers are associated with the outcome of patients with prostate cancer, including the following:[20,21]
- Markers of apoptosis including Bcl-2, Bax.
- Markers of proliferation rate, such as Ki67.
- TP53 mutation or expression.
- p27.
- E-cadherin.
- Microvessel density.
- DNA ploidy.
- p16.
- PTEN gene hypermethylation and allelic losses.
However, none of these has been prospectively validated, and they are not a part of the routine management of patients.
Clinical Presentation
In the United States, most prostate cancers are diagnosed as a result of screening; therefore, symptoms of cancer are infrequent at the time of diagnosis.[21] Nevertheless, local growth of the tumor may produce symptoms of urinary obstruction such as:
- Decreased urinary stream.
- Urgency.
- Hesitancy.
- Nocturia.
- Incomplete bladder emptying.
These symptoms are nonspecific and more indicative of benign prostatic hyperplasia than cancer.
Although rare in the current era of widespread screening, prostate cancer may also present with symptoms of metastases, including bone pain, pathological fractures, or symptoms caused by bone marrow involvement.
Diagnostic Evaluation
Needle biopsy is the most common method used to diagnose prostate cancer. Most urologists now perform a transrectal biopsy using a bioptic gun with ultrasound guidance. Less frequently, a transperineal ultrasound-guided approach can be used in patients who may be at increased risk of complications from a transrectal approach.[25] Over the years, there has been a trend toward taking eight to ten or more biopsy samples from several areas of the prostate with a consequent increased yield of cancer detection after an elevated PSA blood test.[21]
The use of magnetic resonance imaging (MRI)−directed biopsy in the initial diagnostic evaluation of prostate cancer is also being studied, either as a replacement for, or in addition to, standard systematic prostate needle biopsies. The data have been reported primarily by experienced MRI radiologists and urologists in referral centers, and generalizability of results is uncertain. A multicenter randomized trial of 500 patients has shown that, in experienced hands, a multiparametric MRI-directed biopsy is more accurate than a transrectal-guided biopsy to detect clinically significant cancers. MRI led to the detection of more Gleason score (≥7) lesions and fewer Gleason score (<7) lesions, with fewer biopsies overall.[26] The data suggested that MRI-directed biopsy can replace standard transrectal-guided biopsies. However, a large, single-arm, single-center study of 2,103 men with MRI-visible lesions who underwent both MRI-directed biopsies and standard systematic prostate needle biopsies under ultrasound visualization suggested otherwise.[27] In that study, MRI-directed biopsies alone led to misclassification of 8.8% of cancers defined as clinically significant (Gleason score 4 + 3 or higher) compared with the combination of both biopsy techniques. Both studies reported only on histology end points at the time of diagnosis, rather than health outcomes on follow-up.
Prophylactic antibiotics, especially fluoroquinolones, are often used before transrectal needle biopsies. There are reports of increasing rates of sepsis, particularly with fluoroquinolone-resistant E. coli, and hospitalization after the procedure.[28,29] Therefore, men undergoing transrectal biopsy should be told to seek medical attention immediately if they experience fever after biopsy.
Prognostic Factors
The following factors influence the survival of patients with prostate cancer:[30,31,32,33,34]
- Extent of tumor.
- Histological grade of tumor.
- Patient's age and health.
- Prostate-specific antigen (PSA) level.
For more information on survival rates, see Cancer Stat Facts: Prostate Cancer.
Extent of tumor
When the cancer is confined to the prostate gland, long-term prognosis is excellent. Patients with locally advanced cancer are not usually curable, but 5-year survival is still very good. If prostate cancer has spread to distant organs, current therapy will not cure it. Median survival is usually 1 to 3 years, and most of these patients will die of prostate cancer. Even in this group of patients, indolent clinical courses lasting for many years may be observed.
Histological grade of tumor
Poorly differentiated tumors are more likely to have metastasized before diagnosis and are associated with a poorer prognosis. The most commonly used method to report tumor differentiation is the Gleason score. For more information, see the Pathology section.
Patient's age and health
Any benefits of definitive local therapy with curative intent may take years to emerge. Therefore, therapy with curative intent is usually reserved for men with a sufficiently long life expectancy. For example, radical prostatectomy is often reserved for men with an estimated life expectancy of at least 10 years.
Prostate-specific antigen (PSA) level
PSA, an organ-specific marker, is often used as a tumor marker.[32,33,35,36,37,38,39,40] The higher the level of PSA at baseline, the higher the risk of metastatic disease or subsequent disease progression. However, it is an imprecise marker of risk.
For example, baseline PSA and rate of PSA change were associated with subsequent metastasis or prostate cancer death in a cohort of 267 men with clinically localized prostate cancer who were managed by watchful waiting or active surveillance in the control arm of a randomized trial comparing radical prostatectomy with watchful waiting or active surveillance.[41,42] Nevertheless, the accuracy of classifying men into groups whose cancer remained indolent versus those whose cancer progressed was poor at all examined cut points of PSA or PSA rate of change.
Serum acid phosphatase levels
Elevations of serum acid phosphatase are associated with poor prognosis in both localized and disseminated disease. However, serum acid phosphatase levels are not incorporated into the American Joint Committee on Cancer's (AJCC) staging system for prostate cancer.[35]
Use of nomograms as a prognostic tool
Several nomograms have been developed to predict outcomes either before radical prostatectomy [43,44,45,46] or after radical prostatectomy [47,48] with intent to cure. Preoperative nomograms are based on clinical stage, PSA level, Gleason score, and the number of positive and negative prostate biopsy cores. One independently validated nomogram demonstrated increased accuracy in predicting biochemical recurrence-free survival by including preoperative plasma levels of transforming growth factor B1 and interleukin-6 soluble receptor.[49,50]
Postoperative nomograms add pathological findings, such as capsular invasion, surgical margins, seminal vesicle invasion, and lymph node involvement. The nomograms, however, were developed at academic centers and may not be as accurate when generalized to nonacademic hospitals, where most patients are treated.[51,52] In addition, the nomograms use nonhealth (intermediate) outcomes, such as PSA rise or pathological surgical findings, and subjective end points, such as the physician's perceived need for additional therapy. In addition, the nomograms may be affected by changing methods of diagnosis or neoadjuvant therapy.[44]
Follow-Up After Treatment
The optimal follow-up strategy for men treated for prostate cancer is uncertain. Men should be interviewed and examined for symptoms or signs of recurrent or progressing disease, as well as side effects of therapy that can be managed by changes in therapy. However, using surrogate end points for clinical decision-making is controversial, and the evidence that changing therapy based on such end points translates into clinical benefit is weak. Often, rates of PSA change are thought to be markers of tumor progression. However, even though a tumor marker or characteristic may be consistently associated with a high risk of prostate cancer progression or death, it may be a very poor predictor and of very limited utility in making therapeutic decisions.
Although the PSA test is nearly universally used to follow patients, the diversity of recommendations on the provision of follow-up care reflects the current lack of research evidence on which to base firm conclusions. A systematic review of international guidelines highlights the need for robust primary research to inform future evidence-based models of follow-up care for men with prostate cancer.[53]
Preliminary data from a retrospective cohort of 8,669 patients with clinically localized prostate cancer treated with either radical prostatectomy or radiation therapy suggested that short posttreatment PSA doubling time (<3 months in this study) fulfills some criteria as a surrogate end point for all-cause mortality and prostate cancer-specific mortality after surgery or radiation therapy.[54]
Likewise, a retrospective analysis (SWOG-S9916 [NCT00004001]) showed PSA declines of 20% to 40% (but not 50%) at 3 months and 30% or more at 2 months after initiation of chemotherapy for hormone-independent prostate cancer, and fulfilled several criteria of surrogacy for overall survival (OS).[55]
These observations should be independently confirmed in prospective study designs and may not apply to patients treated with hormonal therapy. In addition, there are no standardized criteria of surrogacy or standardized cut points for adequacy of surrogate end points, even in prospective trials.[56]
Follow-up after radical prostatectomy
After radical prostatectomy, a detectable PSA level identifies patients at elevated risk of local treatment failure or metastatic disease;[37] however, a substantial proportion of patients with an elevated or rising PSA level after surgery remain clinically free of symptoms for extended periods.[57] Biochemical evidence of failure on the basis of elevated or slowly rising PSA alone, therefore, may not be sufficient to initiate additional treatment.
For example, in a retrospective analysis of nearly 2,000 men who had undergone radical prostatectomy with curative intent and were followed for a mean of 5.3 years, 315 men (15%) demonstrated an abnormal PSA of 0.2 ng/mL or higher, which is considered evidence of biochemical recurrence. Among these 315 men, 103 (34%) developed clinical evidence of recurrence. The median time to the development of clinical metastasis after biochemical recurrence was 8 years. After the men developed metastatic disease, the median time to death was an additional 5 years.[58]
Follow-up after radiation therapy
For patients treated with radiation therapy, the combination of clinical tumor stage, Gleason score, and pretreatment PSA level is often used to estimate the risk of relapse.[59][Level of evidence C2] As is the case after prostatectomy, PSA is often followed for signs of tumor recurrence after radiation therapy. After radiation therapy with curative intent, persistently elevated or rising PSA may be a prognostic factor for clinical disease recurrence; however, reported case series have used a variety of definitions of PSA failure. Criteria have been developed by the American Society for Therapeutic Radiology and Oncology Consensus Panel.[60,61] It is difficult to base decisions about initiating additional therapy on biochemical failure alone. The implication of the various definitions of PSA failure for OS is not known, and, as in the surgical series, many biochemical relapses (rising PSA only) may not be clinically manifested in patients treated with radiation therapy.[62,63]
Follow-up after hormonal therapy
After hormonal therapy, reduction of PSA to undetectable levels provides information regarding the duration of progression-free status; however, decreases in PSA of less than 80% may not be very predictive.[32] Because PSA expression itself is under hormonal control, androgen deprivation therapy can decrease the serum level of PSA independent of tumor response. Clinicians, therefore, cannot rely solely on the serum PSA level to monitor a patient's response to hormonal therapy; they must also follow clinical criteria.[64]
References:
-
National Cancer Institute: SEER Stat Fact Sheets: Prostate. Bethesda, Md: National Cancer Institute. Available online. Last accessed March 11, 2024.
-
American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024. Available online. Last accessed June 21, 2024.
-
Lu-Yao GL, Albertsen PC, Moore DF, et al.: Outcomes of localized prostate cancer following conservative management. JAMA 302 (11): 1202-9, 2009.
-
Albertsen PC, Moore DF, Shih W, et al.: Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol 29 (10): 1335-41, 2011.
-
Welch HG, Albertsen PC: Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst 101 (19): 1325-9, 2009.
-
Welch HG, Black WC: Overdiagnosis in cancer. J Natl Cancer Inst 102 (9): 605-13, 2010.
-
Zlotta AR, Egawa S, Pushkar D, et al.: Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 105 (14): 1050-8, 2013.
-
Helgesen F, Holmberg L, Johansson JE, et al.: Trends in prostate cancer survival in Sweden, 1960 through 1988: evidence of increasing diagnosis of nonlethal tumors. J Natl Cancer Inst 88 (17): 1216-21, 1996.
-
Berner A, Harvei S, Skjorten FJ: Follow-up of localized prostate cancer, with emphasis on previous undiagnosed incidental cancer. BJU Int 83 (1): 47-52, 1999.
-
Garnick MB: Prostate cancer: screening, diagnosis, and management. Ann Intern Med 118 (10): 804-18, 1993.
-
Croswell JM, Kramer BS, Crawford ED: Screening for prostate cancer with PSA testing: current status and future directions. Oncology (Williston Park) 25 (6): 452-60, 463, 2011.
-
Bill-Axelson A, Holmberg L, Ruutu M, et al.: Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 352 (19): 1977-84, 2005.
-
Wilt TJ, Brawer MK, Jones KM, et al.: Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 367 (3): 203-13, 2012.
-
Hegarty J, Beirne PV, Walsh E, et al.: Radical prostatectomy versus watchful waiting for prostate cancer. Cochrane Database Syst Rev (11): CD006590, 2010.
-
Andriole GL, Grubb RL, Buys SS, et al.: Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 360 (13): 1310-9, 2009.
-
Schröder FH, Hugosson J, Roobol MJ, et al.: Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360 (13): 1320-8, 2009.
-
Sandblom G, Varenhorst E, Rosell J, et al.: Randomised prostate cancer screening trial: 20 year follow-up. BMJ 342: d1539, 2011.
-
Djulbegovic M, Beyth RJ, Neuberger MM, et al.: Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ 341: c4543, 2010.
-
Ilic D, O'Connor D, Green S, et al.: Screening for prostate cancer: an updated Cochrane systematic review. BJU Int 107 (6): 882-91, 2011.
-
Nelson WG, De Marzo AM, Isaacs WB: Prostate cancer. N Engl J Med 349 (4): 366-81, 2003.
-
Zelefsky MJ, Eastham JA, Sartor AO: Cancer of the prostate. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 1220-71.
-
Chan TY, Partin AW, Walsh PC, et al.: Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology 56 (5): 823-7, 2000.
-
Albertsen PC, Hanley JA, Barrows GH, et al.: Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst 97 (17): 1248-53, 2005.
-
Thompson IM, Canby-Hagino E, Lucia MS: Stage migration and grade inflation in prostate cancer: Will Rogers meets Garrison Keillor. J Natl Cancer Inst 97 (17): 1236-7, 2005.
-
Webb JA, Shanmuganathan K, McLean A: Complications of ultrasound-guided transperineal prostate biopsy. A prospective study. Br J Urol 72 (5 Pt 2): 775-7, 1993.
-
Kasivisvanathan V, Rannikko AS, Borghi M, et al.: MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med 378 (19): 1767-1777, 2018.
-
Ahdoot M, Wilbur AR, Reese SE, et al.: MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 382 (10): 917-928, 2020.
-
Nam RK, Saskin R, Lee Y, et al.: Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol 183 (3): 963-8, 2010.
-
Liss MA, Chang A, Santos R, et al.: Prevalence and significance of fluoroquinolone resistant Escherichia coli in patients undergoing transrectal ultrasound guided prostate needle biopsy. J Urol 185 (4): 1283-8, 2011.
-
Gittes RF: Carcinoma of the prostate. N Engl J Med 324 (4): 236-45, 1991.
-
Paulson DF, Moul JW, Walther PJ: Radical prostatectomy for clinical stage T1-2N0M0 prostatic adenocarcinoma: long-term results. J Urol 144 (5): 1180-4, 1990.
-
Matzkin H, Eber P, Todd B, et al.: Prognostic significance of changes in prostate-specific markers after endocrine treatment of stage D2 prostatic cancer. Cancer 70 (9): 2302-9, 1992.
-
Pisansky TM, Cha SS, Earle JD, et al.: Prostate-specific antigen as a pretherapy prognostic factor in patients treated with radiation therapy for clinically localized prostate cancer. J Clin Oncol 11 (11): 2158-66, 1993.
-
Chodak GW, Thisted RA, Gerber GS, et al.: Results of conservative management of clinically localized prostate cancer. N Engl J Med 330 (4): 242-8, 1994.
-
Carlton JC, Zagars GK, Oswald MJ: The role of serum prostatic acid phosphatase in the management of adenocarcinoma of the prostate with radiotherapy. Int J Radiat Oncol Biol Phys 19 (6): 1383-8, 1990.
-
Stamey TA, Yang N, Hay AR, et al.: Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 317 (15): 909-16, 1987.
-
Stamey TA, Kabalin JN: Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. I. Untreated patients. J Urol 141 (5): 1070-5, 1989.
-
Stamey TA, Kabalin JN, McNeal JE, et al.: Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. II. Radical prostatectomy treated patients. J Urol 141 (5): 1076-83, 1989.
-
Stamey TA, Kabalin JN, Ferrari M: Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. III. Radiation treated patients. J Urol 141 (5): 1084-7, 1989.
-
Andriole GL: Serum prostate-specific antigen: the most useful tumor marker. J Clin Oncol 10 (8): 1205-7, 1992.
-
Fall K, Garmo H, Andrén O, et al.: Prostate-specific antigen levels as a predictor of lethal prostate cancer. J Natl Cancer Inst 99 (7): 526-32, 2007.
-
Parekh DJ, Ankerst DP, Thompson IM: Prostate-specific antigen levels, prostate-specific antigen kinetics, and prostate cancer prognosis: a tocsin calling for prospective studies. J Natl Cancer Inst 99 (7): 496-7, 2007.
-
Partin AW, Kattan MW, Subong EN, et al.: Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA 277 (18): 1445-51, 1997.
-
Partin AW, Mangold LA, Lamm DM, et al.: Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology 58 (6): 843-8, 2001.
-
Kattan MW, Eastham JA, Stapleton AM, et al.: A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 90 (10): 766-71, 1998.
-
Stephenson AJ, Scardino PT, Eastham JA, et al.: Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 98 (10): 715-7, 2006.
-
Kattan MW, Wheeler TM, Scardino PT: Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol 17 (5): 1499-507, 1999.
-
Stephenson AJ, Scardino PT, Eastham JA, et al.: Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol 23 (28): 7005-12, 2005.
-
Shariat SF, Walz J, Roehrborn CG, et al.: External validation of a biomarker-based preoperative nomogram predicts biochemical recurrence after radical prostatectomy. J Clin Oncol 26 (9): 1526-31, 2008.
-
Kattan MW, Shariat SF, Andrews B, et al.: The addition of interleukin-6 soluble receptor and transforming growth factor beta1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol 21 (19): 3573-9, 2003.
-
Penson DF, Grossfeld GD, Li YP, et al.: How well does the Partin nomogram predict pathological stage after radical prostatectomy in a community based population? Results of the cancer of the prostate strategic urological research endeavor. J Urol 167 (4): 1653-7; discussion 1657-8, 2002.
-
Greene KL, Meng MV, Elkin EP, et al.: Validation of the Kattan preoperative nomogram for prostate cancer recurrence using a community based cohort: results from cancer of the prostate strategic urological research endeavor (capsure). J Urol 171 (6 Pt 1): 2255-9, 2004.
-
McIntosh HM, Neal RD, Rose P, et al.: Follow-up care for men with prostate cancer and the role of primary care: a systematic review of international guidelines. Br J Cancer 100 (12): 1852-60, 2009.
-
D'Amico AV, Moul JW, Carroll PR, et al.: Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst 95 (18): 1376-83, 2003.
-
Petrylak DP, Ankerst DP, Jiang CS, et al.: Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst 98 (8): 516-21, 2006.
-
Baker SG: Surrogate endpoints: wishful thinking or reality? J Natl Cancer Inst 98 (8): 502-3, 2006.
-
Frazier HA, Robertson JE, Humphrey PA, et al.: Is prostate specific antigen of clinical importance in evaluating outcome after radical prostatectomy. J Urol 149 (3): 516-8, 1993.
-
Pound CR, Partin AW, Eisenberger MA, et al.: Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281 (17): 1591-7, 1999.
-
Pisansky TM, Kahn MJ, Rasp GM, et al.: A multiple prognostic index predictive of disease outcome after irradiation for clinically localized prostate carcinoma. Cancer 79 (2): 337-44, 1997.
-
Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys 37 (5): 1035-41, 1997.
-
Roach M, Hanks G, Thames H, et al.: Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65 (4): 965-74, 2006.
-
Kuban DA, el-Mahdi AM, Schellhammer PF: Prostate-specific antigen for pretreatment prediction and posttreatment evaluation of outcome after definitive irradiation for prostate cancer. Int J Radiat Oncol Biol Phys 32 (2): 307-16, 1995.
-
Sandler HM, Dunn RL, McLaughlin PW, et al.: Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys 48 (3): 629-33, 2000.
-
Ruckle HC, Klee GG, Oesterling JE: Prostate-specific antigen: concepts for staging prostate cancer and monitoring response to therapy. Mayo Clin Proc 69 (1): 69-79, 1994.
Stage Information for Prostate Cancer
Staging Tests
Most men are diagnosed with prostate cancer at an early clinical stage and do not have detectable metastases. Therefore, they generally do not have to undergo staging tests, such as a bone scan, computed tomography (CT), or magnetic resonance imaging (MRI). However, staging studies are done if there is clinical suspicion of metastasis, such as bone pain; local tumor spread beyond the prostate capsule; or a substantial risk of metastasis (prostate-specific antigen [PSA] >20 ng/mL and Gleason score >7).[1]
Tests used to determine stage include the following:
- Serum PSA level.
- MRI.
- Positron emission tomography (PET).
- Gallium Ga 68 (68Ga)-gozetotide and fluorine F 18 (18F)-piflufolastat PET-CT.
- Fluorine F 18 (18F)-fluciclovine PET-CT.
- Pelvic lymph node dissection (PLND).
- Transrectal or transperineal biopsy.
- Transrectal ultrasound (TRUS).
- CT scans.
- Technetium Tc 99m (99mTc)-methylene diphosphonate (MDP) bone scan.
Serum prostate-specific antigen (PSA) level
Serum PSA can predict the results of radionuclide bone scans in newly diagnosed patients.
- In one series, only 2 of 852 patients (0.23%) with a PSA of less than 20 ng/mL had a positive bone scan in the absence of bone pain.[2]
- In another series of 265 patients with prostate cancer, 0 of 23 patients with a PSA of less than 4 ng/mL had a positive bone scan, and 2 of 114 patients with a PSA of less than 10 ng/mL had a positive bone scan.[3]
Magnetic resonance imaging (MRI)
Although MRI has been used to detect extracapsular extension of prostate cancer, a positive-predictive value of about 70% and considerable interobserver variation are problems that make its routine use in staging uncertain.[4] Ultrasound and MRI, however, can reduce clinical understaging and thereby improve patient selection for local therapy. MRI with an endorectal coil appears to be more accurate for identification of organ-confined and extracapsular disease, especially when combined with spectroscopy.[1] MRI is a poor tool for evaluating nodal disease.
MRI is more sensitive than radionuclide bone scans in the detection of bone metastases, but it is impractical for evaluating the entire skeletal system.
Positron emission tomography (PET)
It is becoming more common to use PET-CT with specific radionuclide tracers to stage prostate cancer. Several tracers have been tested and shown the ability to detect either lymph node or distant metastases in certain patients with prostate cancer.
68Ga-gozetotide and 18F-piflufolastat PET-CT
Prostate-specific membrane antigen (PSMA) is a transmembrane receptor expressed in high levels in prostate cancer. PSMA can be targeted for imaging with 68Ga-gozetotide and 18F-piflufolastat. These radionuclide tracers have been tested for the imaging of nodes and metastases in the initial staging of intermediate- and high-risk prostate cancer, as well as imaging of suspected posttreatment recurrent disease in patients with an elevated PSA.
A phase III trial included 764 patients with intermediate- or high-risk prostate cancer who underwent 68Ga-gozetotide PET-CT staging. The trial reported a sensitivity of 40% and a specificity of 95% in the detection of nodal disease as compared with PLND.[5]
68Ga-gozetotide PET-CT was studied alongside CT and bone scan for the detection of metastatic disease in men with high-risk prostate cancer. Compared with conventional imaging, 68Ga-gozetotide PET-CT provided increased sensitivity (85% vs. 38%) and specificity (98% vs. 91%).[6] 68Ga-gozetotide PET-CT was also evaluated to assess recurrent disease and showed a high positive predictive value (PPV) and detection rate.[7] 68Ga-gozetotide also had better results than 18F-fluciclovine in that context.[8]
18F-piflufolastat PET-CT had a sensitivity of 40% and a specificity of 98% in staging intermediate- or high-risk prostate cancer compared with PLND.[9] For the detection of recurrent or metastatic prostate cancer in the context of increasing PSA, 18F-piflufolastat PET-CT had a sensitivity of 95.8% and a PPV of 81.9%.[9]
Based on these data, the U.S. Food and Drug Administration (FDA) approved 68Ga-gozetotide and 18F-piflufolastat PET-CT for the initial staging of patients with prostate cancer and suspicion of metastatic disease, and for the evaluation of potential recurrence based on an elevated posttreatment PSA.[10,11]
18F-fluciclovine PET-CT
18F-fluciclovine PET-CT showed low sensitivity but high specificity in the initial lymph nodal staging of intermediate- and high-risk prostate cancer, compared with PLND.[12,13,14] Compared with conventional imaging, its specificity was similar, but sensitivity was higher for detection of extraprostatic disease.[14]
18F-fluciclovine also detected more bone metastases and was more sensitive and specific than 99mTc-MDP bone scan.[15]
The FDA approved 18F-fluciclovine PET-CT for the assessment of suspected recurrent disease in men with a rising posttreatment PSA.
Pelvic lymph node dissection (PLND)
PLND remains the most accurate method to assess metastasis to the pelvic nodes, and laparoscopic PLND has been shown to accurately assess pelvic nodes as effectively as an open procedure.[16]
The determining factor in deciding whether any type of PLND is indicated is when definitive therapy may be altered. For example, radical prostatectomy is generally reserved for men without lymph node metastasis. Likewise, preoperative seminal vesicle biopsy may be useful in patients with palpable nodules who are being considered for radical prostatectomy (unless they have a low Gleason score) because seminal vesicle involvement could affect the choice of primary therapy and predicts for pelvic lymph node metastasis.[17]
In patients with clinically localized (stage I or stage II) prostate cancer, Gleason pathological grade and enzymatic serum prostatic acid phosphatase values (even within normal range) predict the likelihood of capsular penetration, seminal vesicle invasion, or regional lymph node involvement.[18] Analysis of a series of 166 patients with clinical stage I or stage II prostate cancer undergoing radical prostatectomy revealed an association between Gleason biopsy score and the risk of lymph node metastasis found at surgery. The risks of nodal metastasis for patients grouped according to their Gleason biopsy score was 2% for a Gleason score of 5, 13% for a Gleason score of 6, and 23% for a Gleason score of 8.[19]
Having all patients undergo a PLND is debatable, but in patients undergoing a radical retropubic prostatectomy, nodal status is usually ascertained as a matter of course. Evidence is mounting that PLND is likely unnecessary in patients with a PSA less than 20 ng/mL and a low Gleason score who are undergoing radical perineal prostatectomy. This is especially true for patients whose malignancy was not palpable but detected on ultrasound.[18,20]
Transrectal or transperineal biopsy
The most common means to establish a diagnosis and determine the Gleason score in cases of suspected prostate cancer is by needle biopsy. Most urologists now perform a transrectal biopsy using a bioptic gun with ultrasound guidance. Less frequently, a transperineal ultrasound-guided approach can be used for those patients who may be at increased risk of complications from a transrectal approach.[21] Over the years, there has been a trend toward taking eight to ten or more biopsy samples from several areas of the prostate with a consequent increased yield of cancer detection after an elevated PSA blood test.[1]
Transrectal ultrasound (TRUS)
TRUS may facilitate diagnosis by directing needle biopsy; however, ultrasound is operator dependent and does not assess lymph node size.
A prospective multi-institutional study of preoperative TRUS in men with clinically localized prostate cancer eligible for radical prostatectomy showed that TRUS was no better than digital rectal examination in predicting extracapsular tumor extension or seminal vesicle involvement.[22]
Computed tomography (CT) scans
CT scans can detect grossly enlarged lymph nodes but poorly define intraprostatic features;[23] therefore, it is not reliable for the staging of pelvic node disease when compared with surgical staging.[24]
Technetium Tc 99m (99mTc)-methylene diphosphonate (MDP) bone scan
A 99mTc-MDP bone scan is the most widely used test for metastasis to the bone, which is the most common site of distant tumor spread.
Staging Systems
Historically, two systems have been in common use for the staging of prostate cancer.
- In 1975, the Jewett system (stage A through stage D) was described and has since been modified.[25] This staging system is no longer in common use, but older studies and publications may refer to it.
- In 1997, the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer adopted a revised TNM (tumor, node, metastasis) system, which used the same broad T-stage categories as the Jewett system but included subcategories of T stage, such as a stage to describe patients diagnosed through PSA screening. This revised TNM system more precisely stratifies newly diagnosed patients.
AJCC Stage Groupings and TNM Definitions
The AJCC has designated staging by TNM classification.[26]
Table 1. Definition of Histological Grade Groupa
| Grade Group |
Gleason Score |
Gleason Pattern |
|
a Adapted from AJCC: Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 715–26. |
| 1 |
≤6 |
≤3+3 |
| 2 |
7 |
3+4 |
| 3 |
7 |
4+3 |
| 4 |
8 |
4+4, 3+5, or 5+3 |
| 5 |
9 or 10 |
4+5, 5+4, or 5+5 |
Table 2. Definitions of TNM Stage Ia
| Stage |
TNM |
Descriptionb,c,d,e |
PSAf |
Gleason Score; Gleason Pattern (Grade Group)g |
Illustration |
| T = primary tumor; N = regional lymph nodes; M = distant metastasis; cT = clinical T; PSA = prostate-specific antigen; pT = pathological T. |
|
a Adapted from AJCC: Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 715–26. |
| The explanations for superscripts b through g are at the end of Table 5. |
| I |
cT1a–c, cT2a, N0, M0 |
cT1 = Clinically inapparent tumor that is not palpable. |
<10 |
Gleason Score, ≤6; Gleason Pattern, ≤3+3 (1). |
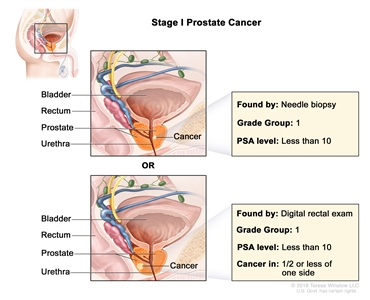
|
| –cT1a = Tumor incidental histological finding in ≤5% of tissue resected. |
| –cT1b = Tumor incidental histological finding in >5% of tissue resected. |
| –cT1c = Tumor identified by needle biopsy found in one or both sides, but not palpable. |
| cT2 = Tumor is palpable and confined within prostate. |
| –cT2a = Tumor involves ½ of one side or less. |
| N0 = No positive regional nodes. |
| M0 = No distant metastasis. |
| pT2, N0, M0 |
pT2 = Organ confined. |
<10 |
Gleason Score, ≤6; Gleason Pattern, ≤3+3 (1). |
| N0 = No positive regional nodes. |
| M0 = No distant metastasis. |
Table 3. Definitions of TNM Stages IIA, IIB, and IICa
| Stage |
TNM |
Descriptionb,c,d,e |
PSAf |
Gleason Score; Gleason Pattern (Grade Group)g |
Illustration |
| T = primary tumor; N = regional lymph nodes; M = distant metastasis; cT = clinical T; PSA = prostate-specific antigen; pT = pathological T. |
|
a Adapted from AJCC: Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 715–26. |
| The explanations for superscripts b through g are at the end of Table 5. |
| IIA |
cT1a–c, cT2a, pT2, N0, M0 |
See cT1a–c, cT2a descriptions in Table 2, Stage I. |
≥10 <20 |
Gleason Score, ≤6; Gleason Pattern, ≤3+3 (1). |
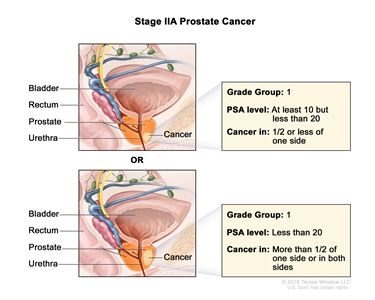
|
| pT2 = Organ confined. |
| cT2b–c, N0, M0 |
cT2 = Tumor is palpable and confined within prostate. |
<20 |
Gleason Score, ≤6; Gleason Pattern, ≤3+3 (1). |
| cT2b = Tumor involves >½ of one side but not both sides. |
| cT2c = Tumor involves both sides. |
| N0 = No positive regional nodes. |
| M0 = No distant metastasis. |
| IIB |
T1–2, N0, M0 |
T1 = Clinically inapparent tumor that is not palpable. |
<20 |
Gleason Score, 7; Gleason Pattern 3+4 (2). |
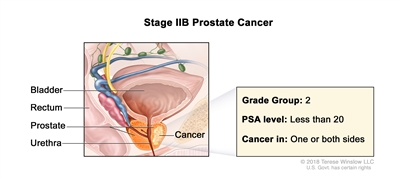
|
| –T1a = Tumor incidental histological finding in ≤5% of tissue resected. |
| –T1b = Tumor incidental histological finding in >5% of tissue resected. |
| –T1c = Tumor identified by needle biopsy found in one or both sides, but not palpable. |
| cT2 = Tumor is palpable and confined within prostate. |
| –cT2a = Tumor involves ½ of one side or less. |
| –cT2b = Tumor involves >½ of one side but not both sides. |
| –cT2c = Tumor involves both sides. |
| pT2 = Organ confined. |
| N0 = No positive regional nodes. |
| M0 = No distant metastasis. |
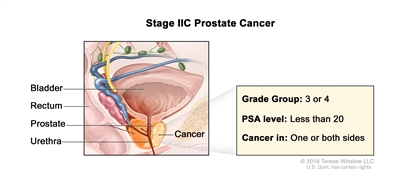
| IIC |
T1–2, N0, M0 |
See T1–2, N0, M0 descriptions above in Stage IIB. |
<20 |
Gleason Score, 7; Gleason Pattern, 4 + 3 (3). |
|
| T1–2, N0, M0 |
See T1–2, N0, M0 descriptions above in Stage IIB. |
<20 |
Gleason Score, 8; Gleason Pattern, 4+4, 3+5, or 5+3 (4). |
Table 4. Definitions of TNM Stages IIIA, IIIB, and IIICa
| Stage |
TNM |
Descriptionb,c,d,e |
PSAf |
Gleason Score; Gleason Pattern (Grade Group)g |
Illustration |
| T = primary tumor; N = regional lymph nodes; M = distant metastasis; cT = clinical T; PSA = prostate-specific antigen; pT = pathological T. |
|
a Adapted from AJCC: Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 715–26. |
| The explanations for superscripts b through g are at the end of Table 5. |
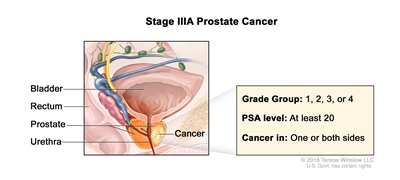
| IIIA |
T1–2, N0, M0 |
See T1–2, N0, M0 descriptions in Table 3, Stage IIB. |
≥20 |
Gleason Score, ≤6; Gleason Pattern, ≤3+3 (1). |
|
| Gleason Score, 7; Gleason Pattern 3+4 (2). |
| Gleason Score, 7; Gleason Pattern, 4+3 (3). |
| Gleason Score, 8; Gleason Pattern, 4+4, 3+5, or 5+3 (4). |
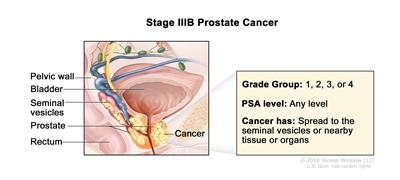
| IIIB |
T3–4, N0, M0 |
cT3 = Extraprostatic tumor that is not fixed or does not invade adjacent structures. |
Any value |
Gleason Score, ≤6; Gleason Pattern, ≤3+3 (1). |
|
| –cT3a = Extraprostatic extension (unilateral or bilateral). |
Gleason Score, 7; Gleason Pattern 3+4 (2). |
| –cT3b = Tumor invades seminal vesicle(s). |
Gleason Score, 7; Gleason Pattern, 4+3 (3). |
| pT3 = Extraprostatic extension. |
Gleason Score, 8; Gleason Pattern, 4+4, 3+5, or 5+3 (4). |
| –pT3a = Extraprostatic extension (unilateral or bilateral) or microscopic invasion of bladder neck. |
| –pT3b = Tumor invades seminal vesicle(s). |
| cT4 or pT4= Tumor is fixed or invades adjacent structures other than seminal vesicles such as external sphincter, rectum, bladder, levator muscles, and/or pelvic wall. |
| N0 = No positive regional nodes. |
| M0 = No distant metastasis. |
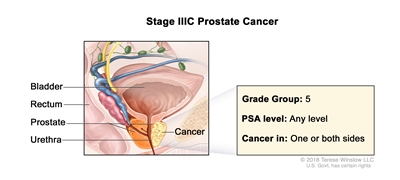
| IIIC |
Any T, N0, M0 |
TX = Primary tumor cannot be assessed. |
Any value |
Gleason Score, 9 or 10; Gleason Pattern, 4+5, 5+4, or 5+5 (5). |
|
| T0 = No evidence of primary tumor. |
| T1 = Clinically inapparent tumor that is not palpable. |
| –T1a = Tumor incidental histological finding in ≤5% of tissue resected. |
| –T1b = Tumor incidental histological finding in >5% of tissue resected. |
| –T1c = Tumor identified by needle biopsy found in one or both sides, but not palpable. |
| cT2 = Tumor is palpable and confined within prostate. |
| –cT2a = Tumor involves ½ of one side or less. |
| –cT2b = Tumor involves >½ of one side but not both sides. |
| –cT2c = Tumor involves both sides. |
| –pT2 = Organ confined. |
| cT3 = Extraprostatic tumor that is not fixed or does not invade adjacent structures. |
| –cT3a = Extraprostatic extension (unilateral or bilateral). |
| –cT3b = Tumor invades seminal vesicle(s). |
| pT3 = Extraprostatic extension. |
| –pT3a = Extraprostatic extension (unilateral or bilateral) or microscopic invasion of bladder neck. |
| –pT3b = Tumor invades seminal vesicle(s). |
| cT4 or pT4 = Tumor is fixed or invades adjacent structures other than seminal vesicles such as external sphincter, rectum, bladder, levator muscles, and/or pelvic wall. |
| N0 = No positive regional nodes. |
| M0 = No distant metastasis. |
Table 5. Definitions of TNM Stages IVA and IVBa
| Stage |
TNM |
Descriptionb,c,d,e |
PSAf |
Gleason Score; Gleason Pattern (Grade Group)g |
Illustration |
| T = primary tumor; N = regional lymph nodes; M = distant metastasis; cT = clinical T; PSA = prostate-specific antigen; pT = pathological T. |
|
a Adapted from AJCC: Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 715–26. |
|
b When either PSA or Grade Group is not available, grouping should be determined by T category and/or either PSA or Grade Group as available. |
|
c There is no pathological T1 classification. |
|
d Positive surgical margin should be indicated by an R1 descriptor, indicating residual microscopic disease. |
|
e When more than one site of metastasis is present, the most advanced category is used. M1c is most advanced. |
|
f PSA values are used to assign this category. |
|
g Recently the Gleason system has been compressed into so-called Grade Groups.[27] |
| IVA |
Any T, N1, M0 |
Any T = See descriptions in Table 4, Stage IIIC. |
See Any PSA values in Table 4, Stage IIIC. |
Gleason Score, ≤6; Gleason Pattern, ≤3+3 (1). |
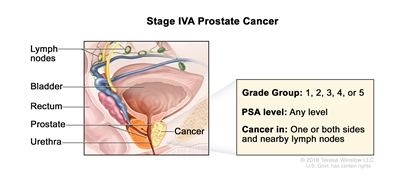
|
| Gleason Score, 7; Gleason Pattern 3+4 (2). |
| Gleason Score, 7; Gleason Pattern, 4+3 (3). |
| N1 = Metastases in regional node(s). |
Gleason Score, 8; Gleason Pattern, 4+4, 3+5, or 5+3 (4). |
| M0 = No distant metastasis. |
Gleason Score, 9 or 10; Gleason Pattern, 4+5, 5+4, or 5+5 (5). |
| IVB |
Any T, Any N, M1 |
Any T = See descriptions in Table 4, Stage IIIC. |
See Any PSA values Table 4, Stage IIIC. |
Any Gleason Score; Gleason Pattern (Grade Group) = See above in Stage IVA. |
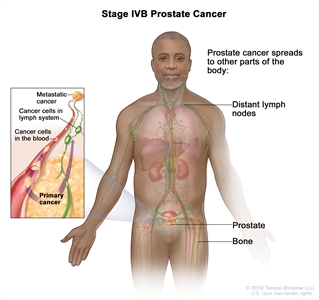
|
| NX = Regional nodes were not assessed. |
| N0 = No positive regional nodes. |
| N1 = Metastases in regional node(s). |
| M1 = Distant metastasis. |
| –M1a = Nonregional lymph node(s). |
| –M1b = Bone(s). |
| –M1c = Other site(s) with or without bone disease. |
References:
-
Zelefsky MJ, Eastham JA, Sartor AO: Cancer of the prostate. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 1220-71.
-
Oesterling JE, Martin SK, Bergstralh EJ, et al.: The use of prostate-specific antigen in staging patients with newly diagnosed prostate cancer. JAMA 269 (1): 57-60, 1993.
-
Huncharek M, Muscat J: Serum prostate-specific antigen as a predictor of radiographic staging studies in newly diagnosed prostate cancer. Cancer Invest 13 (1): 31-5, 1995.
-
Schiebler ML, Yankaskas BC, Tempany C, et al.: MR imaging in adenocarcinoma of the prostate: interobserver variation and efficacy for determining stage C disease. AJR Am J Roentgenol 158 (3): 559-62; discussion 563-4, 1992.
-
Hope TA, Eiber M, Armstrong WR, et al.: Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol 7 (11): 1635-1642, 2021.
-
Hofman MS, Lawrentschuk N, Francis RJ, et al.: Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395 (10231): 1208-1216, 2020.
-
Fendler WP, Calais J, Eiber M, et al.: Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 5 (6): 856-863, 2019.
-
Calais J, Ceci F, Eiber M, et al.: 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol 20 (9): 1286-1294, 2019.
-
Pienta KJ, Gorin MA, Rowe SP, et al.: A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol 206 (1): 52-61, 2021.
-
U.S. Food and Drug Administration: FDA approves first PSMA-targeted PET imaging drug for men with prostate cancer. Food and Drug Administration, 2020. Available online. Last accessed March 11, 2024.
-
U.S. Food and Drug Administration: FDA approves second PSMA-targeted PET imaging drug for men with prostate cancer. Food and Drug Administration, 2021. Available online. Last accessed March 11, 2024.
-
Selnæs KM, Krüger-Stokke B, Elschot M, et al.: 18F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur Radiol 28 (8): 3151-3159, 2018.
-
Suzuki H, Jinnouchi S, Kaji Y, et al.: Diagnostic performance of 18F-fluciclovine PET/CT for regional lymph node metastases in patients with primary prostate cancer: a multicenter phase II clinical trial. Jpn J Clin Oncol 49 (9): 803-811, 2019.
-
Alemozaffar M, Akintayo AA, Abiodun-Ojo OA, et al.: [18F]Fluciclovine Positron Emission Tomography/Computerized Tomography for Preoperative Staging in Patients with Intermediate to High Risk Primary Prostate Cancer. J Urol 204 (4): 734-740, 2020.
-
Chen B, Wei P, Macapinlac HA, et al.: Comparison of 18F-Fluciclovine PET/CT and 99mTc-MDP bone scan in detection of bone metastasis in prostate cancer. Nucl Med Commun 40 (9): 940-946, 2019.
-
Schuessler WW, Pharand D, Vancaillie TG: Laparoscopic standard pelvic node dissection for carcinoma of the prostate: is it accurate? J Urol 150 (3): 898-901, 1993.
-
Stone NN, Stock RG, Unger P: Indications for seminal vesicle biopsy and laparoscopic pelvic lymph node dissection in men with localized carcinoma of the prostate. J Urol 154 (4): 1392-6, 1995.
-
Oesterling JE, Brendler CB, Epstein JI, et al.: Correlation of clinical stage, serum prostatic acid phosphatase and preoperative Gleason grade with final pathological stage in 275 patients with clinically localized adenocarcinoma of the prostate. J Urol 138 (1): 92-8, 1987.
-
Fournier GR, Narayan P: Re-evaluation of the need for pelvic lymphadenectomy in low grade prostate cancer. Br J Urol 72 (4): 484-8, 1993.
-
Daniels GF, McNeal JE, Stamey TA: Predictive value of contralateral biopsies in unilaterally palpable prostate cancer. J Urol 147 (3 Pt 2): 870-4, 1992.
-
Webb JA, Shanmuganathan K, McLean A: Complications of ultrasound-guided transperineal prostate biopsy. A prospective study. Br J Urol 72 (5 Pt 2): 775-7, 1993.
-
Smith JA, Scardino PT, Resnick MI, et al.: Transrectal ultrasound versus digital rectal examination for the staging of carcinoma of the prostate: results of a prospective, multi-institutional trial. J Urol 157 (3): 902-6, 1997.
-
Gerber GS, Goldberg R, Chodak GW: Local staging of prostate cancer by tumor volume, prostate-specific antigen, and transrectal ultrasound. Urology 40 (4): 311-6, 1992.
-
Hanks GE, Krall JM, Pilepich MV, et al.: Comparison of pathologic and clinical evaluation of lymph nodes in prostate cancer: implications of RTOG data for patient management and trial design and stratification. Int J Radiat Oncol Biol Phys 23 (2): 293-8, 1992.
-
Jewett HJ: The present status of radical prostatectomy for stages A and B prostatic cancer. Urol Clin North Am 2 (1): 105-24, 1975.
-
Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 715–26.
-
Epstein JI, Egevad L, Amin MB, et al.: The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 40 (2): 244-52, 2016.
Treatment Option Overview for Prostate Cancer
Local treatment modalities are associated with prolonged disease-free survival (DFS) for many patients with localized prostate cancer but are rarely curative in patients with locally extensive tumors. Because of clinical understaging using current diagnostic techniques, even when the cancer appears clinically localized to the prostate gland, some patients develop disseminated tumors after local therapy with surgery or radiation.
Treatment options for each stage of prostate cancer are presented in Table 6.
Table 6. Treatment Options by Stage for Prostate Cancer
| Stage ( TNM Definitions) |
Treatment Options |
| EBRT = external-beam radiation therapy; LH-RH = luteinizing hormone-releasing hormone; PARP = poly (ADP-ribose) polymerase; TURP = transurethral resection of the prostate. |
| Stage I Prostate Cancer |
Watchful waiting or active surveillance/active monitoring |
| Radical prostatectomy |
| External-beam radiation therapy (EBRT) |
| Interstitial implantation of radioisotopes |
| Photodynamic therapy(under clinical evaluation) |
| Bicalutamide(under clinical evaluation) |
| Stage II Prostate Cancer |
Watchful waiting or active surveillance/active monitoring |
| Radical prostatectomy |
| EBRT with or without hormonal therapy |
| Interstitial implantation of radioisotopes |
| Cryosurgery(under clinical evaluation) |
| Proton-beam therapy(under clinical evaluation) |
| Photodynamic therapy(under clinical evaluation) |
| Neoadjuvant hormonal therapy(under clinical evaluation) |
| Bicalutamide(under clinical evaluation) |
| Stage III Prostate Cancer |
EBRT with or without hormonal therapy |
| Hormonal manipulations with or without radiation therapy |
| Radical prostatectomy with or without EBRT |
| Watchful waiting or active surveillance/active monitoring |
| Cryosurgery(under clinical evaluation) |
| Proton-beam therapy(under clinical evaluation) |
| Bicalutamide(under clinical evaluation) |
| Stage IV Prostate Cancer |
Hormonal manipulations |
| Bisphosphonates |
| EBRT with or without hormonal therapy |
| Palliative radiation therapy |
| Palliative surgery with transurethral resection of the prostate (TURP) |
| Watchful waiting or active surveillance/active monitoring |
| Recurrent Prostate Cancer |
Hormone therapy |
| Chemotherapy for hormone-resistant prostate cancer |
| Immunotherapy |
| Radiopharmaceutical therapy/alpha emitter radiation |
| PARP inhibitors for men with prostate cancer and BRCA1, BRCA2, and/or ATM mutations |
| Cryosurgery(under clinical evaluation) |
Side effects of each of the treatment approaches are covered in the relevant sections below. Patient-reported adverse effects differ substantially across the options for management of clinically localized disease, with few direct comparisons, and include watchful waiting/active surveillance/active monitoring, radical prostatectomy, and radiation therapy. The differences in adverse effects can play an important role in patient choice among treatment options. Detailed comparisons of these effects have been reported in population-based cohort studies, albeit with relatively short follow-up times of 2 to 3 years.[1,2]
Watchful Waiting or Active Surveillance/Active Monitoring
Asymptomatic patients of advanced age or with concomitant illness may warrant consideration of careful observation without immediate active treatment.[3,4] Watch and wait, observation, expectant management, and active surveillance/active monitoring are terms indicating a strategy that does not employ immediate therapy with curative intent.
Watchful waiting and active surveillance/active monitoring are the most commonly used terms, and the literature does not always clearly distinguish them, making the interpretation of results difficult. The general concept of watchful waiting is patient follow-up with the application of palliative care as needed to alleviate symptoms of tumor progression. There is no planned attempt at curative therapy at any point in follow-up. For example, transurethral resection of the prostate (TURP) or hormonal therapy may be used to alleviate tumor-related urethral obstruction should there be local tumor growth; hormonal therapy or bone radiation might be used to alleviate pain from metastases. Radical prostatectomy has been compared with watchful waiting or active surveillance/active monitoring in men with early-stage disease (i.e., clinical stages T1b, T1c, or T2).[5] For more information, see the Radical Prostatectomy section.
In contrast, the strategy behind active surveillance/active monitoring is to defer therapy for clinically localized disease but regularly follow the patient and initiate local therapy with curative intent if there are any signs of local tumor progression.[6,7,8,9] The intention is to avoid the morbidity of therapy in men who have indolent or nonprogressive disease but preserve the ability to cure them should the tumor progress. Active surveillance/active monitoring often involves the following:
- Regular patient visits.
- Digital rectal examinations.
- Prostate-specific antigen (PSA) testing.
- Transrectal ultrasound (in some series).
- Transrectal needle biopsies (in some series).
Patient selection, testing intervals, and specific tests, as well as criteria for intervention, are arbitrary and not established in controlled trials.
In the United States, as in other settings with widespread PSA screening, the results of conservative management of localized prostate cancer are particularly favorable. In the aggregate, men managed by watchful waiting or active surveillance/active monitoring (using various criteria, depending upon the study) have had very favorable prostate–cancer-specific mortalities ranging from about 1% to 10% (with the most favorable rates in more recent series).[10,11,12,13,14,15,16,17,18] Most men with screen-detected prostate cancer may, therefore, be candidates for active surveillance/active monitoring, with definitive therapy reserved for signs of tumor progression. This has been shown most clearly in the large Prostate Testing for Cancer Treatment (ProtecT [NCT02044172 and ISRCTN20141297]) randomized trial that compared active monitoring, radical prostatectomy, and radiation therapy.[19] For more information, see the Radical Prostatectomy section.
For more information, see the Treatment of Stage II Prostate Cancer section.
Radical Prostatectomy
A radical prostatectomy is usually reserved for patients who:[20,21,22]
- Are in good health and elect surgical intervention.
- Have tumor confined to the prostate gland (stage I and stage II).
Open prostatectomy can be performed by the perineal or retropubic approach. The perineal approach requires a separate incision for lymph node dissection. Laparoscopic lymphadenectomy is technically possible.[23] Robot-assisted prostatectomy is an alternative to open prostatectomy and has become the most common technique in developed countries. In experienced hands, functional outcomes between open and robot-assisted prostatectomy appear very similar, at least in the short- to mid-term. In a randomized trial of 308 men suitable for prostatectomy, urinary, sexual, and bowel functional outcomes were similar between open retropubic and robotic surgeries at a median follow-up of 24 months.[24] The sample size and duration of follow-up were too small to detect meaningful differences in cancer outcomes.
For small, well-differentiated nodules, the incidence of positive pelvic nodes is less than 20%, and pelvic node dissection may be omitted.[25] With larger, less-differentiated tumors, a pelvic lymph node dissection is more important. In these cases, the value of open surgical or laparoscopic pelvic node dissection is not therapeutic, but it spares patients with positive nodes the morbidity of prostatectomy. Radical prostatectomy is usually not performed if a frozen-section evaluation of pelvic nodes reveals metastases; these patients should be considered for entry into existing clinical trials or receive radiation therapy to control local symptoms.
The role of preoperative (neoadjuvant) hormonal therapy is not established.[26,27]
After radical prostatectomy, pathological evaluation stratifies tumor extent into the following classes:
- Margin-positive disease—The incidence of disease recurrence increases when the tumor margins are positive.[10,28,29] Results of the outcome of patients with positive surgical margins have not been systematically reported.
- Specimen-confined disease—The incidence of disease recurrence increases when the tumor is not specimen-confined (extracapsular).[10,28]
- Organ-confined disease—Patients with extraprostatic disease (not organ-confined) are suitable candidates for clinical trials of which the Radiation Therapy Oncology Group's (RTOG) RTOG-9601 trial (NCT00002874), was an example. These trials have included evaluation of postoperative radiation delivery, cytotoxic agents, and hormonal treatment using luteinizing hormone-releasing hormone (LH-RH) agonists and/or antiandrogens.
Radical prostatectomy compared with other treatment options
In 1993, a structured literature review of 144 papers was done in an attempt to compare the three primary treatment strategies for clinically localized prostate cancer:[30]
- Radical prostatectomy.
- Definitive radiation therapy.
- Observation (watchful waiting or active surveillance/active monitoring).
The authors concluded that poor reporting and selection factors within all series precluded a valid comparison of efficacy for the three management strategies.
In a literature review of case series of patients with palpable, clinically localized disease, the authors found that 10-year prostate−cancer-specific survival rates were best in radical prostatectomy series (about 93%), worst in radiation therapy series (about 75%), and intermediate with deferred treatment (about 85%).[31] Because it is highly unlikely that radiation therapy would worsen disease-specific survival, the most likely explanation is that selection factors affect choice of treatment. Such selection factors make comparisons of therapeutic strategies imprecise.[32]
Radical prostatectomy has been compared with watchful waiting or active surveillance/active monitoring in men with early-stage disease (i.e., clinical stages T1b, T1c, or T2) in randomized trials, with conflicting results. The difference in results may be the result of differences in how the men were diagnosed with prostate cancer.
Evidence (radical prostatectomy vs. watchful waiting or active surveillance/active monitoring):
- In a randomized clinical trial performed in Sweden in the pre-PSA screening era, 695 men with prostate cancer were randomly assigned to radical prostatectomy versus watchful waiting. Only about 5% of the men in the trial had been diagnosed by PSA screening. Therefore, the men had more extensive local disease than is typically the case in men diagnosed with prostate cancer in the United States.[33,34,35]
- The cumulative overall mortality at 18 years was 56.1% in the radical prostatectomy arm and 68.9% in the watchful waiting study arm (absolute difference, 12.7%; 95% confidence interval [CI], 5.1–20.3 percentage points; relative risk [RR]death, 0.71; 95% CI, 0.59–0.86).[35][Level of evidence A1]
- The cumulative incidence of prostate cancer deaths at 18 years was 17.7% versus 28.7% (absolute difference, 11.0%; 95% CI, 4.5–17.5 percentage points; RRdeath from prostate cancer, 0.56; 95% CI, 0.41–0.77).[35]
- In a post-hoc–subset analysis, the improvement in overall and prostate cancer-specific mortality associated with radical prostatectomy was restricted to men younger than 65 years.
- The Prostate Intervention Versus Observation Trial (PIVOT-1 or VA-CSP-407) is a randomized trial conducted in the PSA screening era that directly compared radical prostatectomy with watchful waiting. From November 1994 through January 2002, 731 men aged 75 years or younger with localized prostate cancer (stage T1–2, NX, M0, with a blood PSA <50 ng/mL) and a life expectancy of at least 10 years were randomly assigned to radical prostatectomy or watchful waiting.[5,36,37][Level of evidence A1]
- About 50% of the men had nonpalpable, screen-detected disease.
- After a median follow-up of 12.7 years (range up to about 19.5 years), the all-cause mortality was 61.3% in the prostatectomy arm versus 66.8% in the watchful-waiting study arm, with an absolute difference of 5.5 percentage points (95% CI, -1.5–12.4) that was not statistically significant (hazard ratio [HR], 0.84; 95% CI, 0.70–1.01). Prostate cancer-specific mortality was 7.4% versus 11.4%, and it also was not statistically significant (HR, 0.63; 95% CI, 0.3–1.02).
- Although treatment for disease progression was given more frequently in the observation arm of the study, most of the treatment was for asymptomatic, local, or biochemical (PSA) progression.
- As expected, urinary incontinence and erectile/sexual dysfunction was more common in the prostatectomy group during at least 10 years of follow-up. Absolute differences in patient-reported use of absorbent urinary pads was greater in the surgery group by more than 30 percentage points at all time points for at least 10 years. Disease- or treatment-related limitations in activities of daily living were worse with surgery than with observation through 2 years, but then were similar in both study arms.
- In the ProtecT trial (NCT02044172 and ISRCTN20141297), 82,429 men were screened with PSA testing, and 2,664 were diagnosed with clinically localized prostate cancer. Among those diagnosed, 1,643 men (median age 62 years, range 50–69 years) consented to a randomly assigned comparison of active monitoring, radical prostatectomy (nerve-sparing when possible), or external-beam 3-dimensional (3D) conformal radiation therapy (74 Gy in 37 fractions). The primary end point was prostate cancer–specific mortality.[19]
- With a median follow-up of 10 years, there were 17 deaths from prostate cancer, with no statistically significant differences among the three study arms (P = .48). The 10-year prostate cancer–specific survival rates were 98.8% in the active monitoring arm, 99.0% in the radical prostatectomy arm, and 99.6% in the radiation therapy arm.[19][Level of evidence A1]
- Likewise, all-cause mortality was nearly identical in all three study arms: 10.9 deaths in the active monitoring arm, 10.1 in the radical prostatectomy arm, and 10.3 in the radiation therapy arm per 1,000 person-years (P = .87).[19][Level of evidence A1]
- There were statistically significant differences in progression to metastatic disease among the treatment arms (33 of 545 men in the active monitoring arm; 13 of 553 men in the radical prostatectomy arm; 16 of 545 men in the radiation therapy arm) that began to emerge after 4 years, but these differences had not translated into any difference in mortality after 10 years of follow-up. Over the course of 10 years, 52% of the patients required active intervention.
- As expected, there were substantial differences in patient-reported outcomes among the three management approaches.[38][Level of evidence A3] A substudy of patient-reported outcomes up to 6 years after randomization included the following:
- Men in the radical prostatectomy study arm had substantial rates of urinary incontinence (e.g., using one or more absorbent pads qd was reported by 46% at 6 months and by 17% at year 6) with very little incontinence in the other two study arms.
- Sexual function was also worse in the radical prostatectomy group (e.g., at 6 months, 12% of men reported erections firm enough for intercourse versus 22% in the radiation therapy arm and 52% in the active monitoring arm).
- Bowel function, however, was worse in the radiation therapy arm (e.g., about 5% reported bloody stools at least half the time at 2 years and beyond vs. none in the radical prostatectomy and active-monitoring study arms).
Complications of radical prostatectomy
Complications of radical prostatectomy include the following:
- Morbidity and mortality associated with general anesthesia and a major surgical procedure.[39,40,41]
- Urinary incontinence and impotence.[42,43,44,45,46,47,48,49]
- Penile shortening.[50,51,52]
- Inguinal hernia.[53,54,55,56,57]
- Fecal incontinence.[58]
Functional outcomes of radical prostatectomy with respect to sexual, urinary, bowel function, and health-related quality of life (QOL), appear to be similar whether the procedure is open retropubic, laparoscopic, or robot-assisted radical prostatectomy.[59]
Morbidity and mortality associated with radical prostatectomy
An analysis of Medicare records on 101,604 radical prostatectomies performed from 1991 to 1994 showed the following:[39]
- A 30-day operative mortality rate of 0.5%.
- A rehospitalization rate of 4.5%.
- A major complication rate of 28.6%.
Over the study period, these rates decreased by 30%, 8%, and 12%, respectively.[39]
Prostatectomies done at hospitals where fewer of the procedures were performed than those done at hospitals where more were performed were associated with the following:[40,41]
- Higher rates of 30-day postoperative mortality.
- Major acute surgical complications.
- Longer hospital stays.
- Higher rates of rehospitalization.
Operative morbidity and mortality rates increase with age. Comorbidity, especially underlying cardiovascular disease and a history of stroke, accounts for a portion of the age-related increase in 30-day mortality.
In a cohort of all men with prostate cancer who underwent radical prostatectomy from 1990 to 1999 in Ontario, 75-year-old men with no comorbidities had a predicted 30-day mortality of 0.74%. Thirty-day surgical complication rates also depended more on comorbidity than age (i.e., about 5% vs. 40% for men with 0 vs. ≥4 underlying comorbid conditions, respectively).[41]
Urinary incontinence and impotence
Urinary incontinence and impotence are complications that can result from radical prostatectomy and have been studied in multiple studies.
Evidence (urinary incontinence and impotence after radical prostatectomy):
- A large case series of men undergoing the anatomic (nerve-sparing) technique of radical prostatectomy reported the following:[43]
- Approximately 6% of the men required the use of pads for urinary incontinence, but an unknown additional proportion of men had occasional urinary dribbling.
- About 40% to 65% of the men who were sexually potent before surgery retained potency adequate for vaginal penetration and sexual intercourse. Preservation of potency with this technique is dependent on tumor stage and patient age, but the operation probably induces at least a partial deficit in nearly all patients.
- A national survey of Medicare patients who underwent radical prostatectomy in 1988 to 1990 reported more morbidity than in the case series reported above.[44]
- More than 30% of the men reported the need for pads or clamps for urinary wetness, and 63% of all patients reported a current problem with wetness.
- About 60% of the men reported having no erections since surgery; about 90% of the men had no erections sufficient for intercourse during the month before the survey.
- About 28% of the patients reported follow-up treatment of cancer with radiation therapy and/or hormonal therapy within 4 years after their prostatectomy.
- A population-based longitudinal cohort (Prostate Cancer Outcomes Study) of 901 men aged 55 to 74 years who had recently undergone radical prostatectomy for prostate cancer reported the following:[45]
- 15.4% of the men had either frequent urinary incontinence or no urinary control at 5 years after surgery.
- 20.4% of those studied wore pads to stay dry.
- 79.3% of men reported an inability to have an erection sufficient for intercourse.
- A cross-sectional survey of patients with prostate cancer who were treated with radical prostatectomy, radiation therapy, or watchful waiting and active surveillance in a managed care setting showed substantial sexual and urinary dysfunction in the prostatectomy group.[46]
- Results reported by the patients were consistent with those from the national Medicare survey.
- In addition, although statistical power was limited, differences in sexual and urinary dysfunction between men who had undergone either nerve-sparing or standard radical prostatectomy were not statistically significant. This issue requires more study.
- Case series of 93, 459, and 89 men who had undergone radical prostatectomy by experienced surgeons showed rates of impotence as high as those in the national Medicare survey when men were carefully questioned about sexual potency, although the men in these case series were on average younger than those in the Medicare survey.[47,48,49] One of the case series used the same questionnaire as that used in the Medicare survey.[47] The urinary incontinence rate in that series was also similar to that in the Medicare survey.
Differences are often reported between population-based surveys and case series from individual centers. Reasons could include the following:
- Age differences among the populations.
- Surgical expertise at the major reporting centers.
- Patient selection factors.
- Publication bias of favorable series.
- Different methods of collecting information from patients.
Penile shortening
Case series of men who have undergone radical prostatectomy have shown shortening of penile length (by an average of 1–2 cm).[50,51,52] The functional consequence of the shortening is not well studied, but it is noticeable to some men.
In a registry of men with rising PSA after initial treatment of clinically localized prostate cancer, 19 of 510 men (3.7%) who had undergone radical prostatectomy complained of reduced penile size.[60] However, the data were based upon physician reporting of patients' complaints rather than direct patient questioning or before-and-after measurement of penile length. Also, the study sample was restricted to patients with known or suspected tumor recurrence, making generalization difficult.
Recovery of penile length to preoperative measurements within 1 to 2 years has been reported in some, but not all, case series in which men were followed longitudinally.[61]
Inguinal hernia
Inguinal hernia has been reported as a complication of radical prostatectomy.
Evidence (inguinal hernia after radical prostatectomy):
- Retrospective cohort studies and case series have shown an increased incidence of inguinal hernia, ranging from 7% to 21%, in men undergoing radical prostatectomy, with rates peaking within 2 years of surgery.[53,54,55,56,57]
- Observational studies suggest that the rates are higher than in comparable men who have undergone prostate biopsy alone, transurethral resections, and simple open prostatectomy for benign disease;[53,54] or in men with prostate cancer who have undergone pelvic lymph node dissection alone or radiation therapy.[53,55,56]
Although the observations of increased rates of inguinal hernia after radical prostatectomy are consistent, it is conceivable that men with prostate cancer who are being followed carefully by urologists could have higher detection rates of hernia because of frequent examinations or diagnostic imaging (i.e., detection bias). Men should be made aware of this potential complication of prostatectomy.
Fecal incontinence
Radical prostatectomy may cause fecal incontinence, and the incidence may vary with surgical method.[58]
Evidence (fecal incontinence after radical prostatectomy):
- In a national survey sample of 907 men who had undergone radical prostatectomy at least 1 year before the survey, 32% of the men who had undergone perineal (nerve-sparing) radical prostatectomy and 17% of the men who had undergone a retropubic radical prostatectomy reported accidents of fecal leakage. Ten percent of the respondents reported moderate amounts of fecal leakage, and 4% of the respondents reported large amounts of fecal leakage. Fewer than 15% of men with fecal incontinence had reported it to a physician or health care provider.[58]
Radiation Therapy and Radiopharmaceutical Therapy
External-beam radiation therapy (EBRT)
Candidates for definitive radiation therapy must have a confirmed pathological diagnosis of cancer that is clinically confined to the prostate and/or surrounding tissues (stage I, stage II, and stage III). Staging laparotomy and lymph node dissection are not required.
Radiation therapy may be a good option for patients who are considered poor medical candidates for radical prostatectomy. These patients can be treated with an acceptably low complication rate if care is given to the delivery technique.[62]
Long-term results with radiation therapy are dependent on stage and are associated with dosimetry of the radiation.
Evidence (EBRT):
- A retrospective review of 999 patients treated with megavoltage radiation therapy showed that cause-specific survival rates at 10 years varied substantially by T stage: T1 (79%), T2 (66%), T3 (55%), and T4 (22%).[63] An initial serum PSA level higher than 15 ng/mL is a predictor of probable failure with conventional radiation therapy.[64]
- Several randomized studies have demonstrated an improvement in freedom from biochemical (PSA-based) recurrence with higher doses of radiation therapy (74–79 Gy) as compared with lower doses (64–70 Gy).[65,66,67,68,69][Level of evidence B1] None of the studies demonstrated a cause-specific survival benefit to higher doses.
- The MRC-RT01 study (NCT00003290) enrolled 843 men with stage T1b through T3a, N0, M0 prostate cancer. Patients were randomly assigned to receive 64 Gy in 32 fractions versus 74 Gy in 37 fractions by conformal delivery.[68] Men in both study groups received neoadjuvant LH-RH agonist injections every 4 weeks for 3 to 6 months before the start of radiation therapy and throughout the radiation course. The study was powered to detect differences in both biochemical progression-free survival (PFS) and a 15% difference in overall survival (OS).
- After a median follow-up of 10 years, despite a statistically significant improvement in biochemical PFS with the higher dose of radiation, the 10-year OS rate was the same in both groups: 71% (HR, 0.99; 95% CI, 0.77–1.28; P = .96). Likewise, there were no differences in prostate—cancer-specific survival.
- Likewise, in the RTOG-0126 trial (NCT00033631), 1,532 men with stage cT1b to T2b (Gleason score 2 to 6 and PSA 10 to <20 ng/mL or Gleason score 7 and PSA <15 ng/mL) prostate cancer were randomly assigned to receive 79.2 Gy in 44 fractions compared with 70.2 Gy in 39 fractions (using 3D conformal or intensity-modulated radiation therapy [IMRT]).[69] With a median follow-up of 8.4 years (maximum, 13.0 years), 8-year OS rates were 76% and 75% (HR, 1.00; 95% CI, 0.83–1.20; P = .98). However, the high-dose radiation was associated with increased late-grade 2 or greater gastrointestinal and genitourinary toxicities (21% and 12% with 79.2 Gy and 15% and 7% with 70.2 Gy).
For more information, see the Radical prostatectomy compared with other treatment options section.
Prophylactic radiation therapy to clinically or pathologically uninvolved pelvic lymph nodes does not appear to improve OS or prostate cancer-specific survival as was seen in the RTOG-7706 trial, for example.[70][Level of evidence A1]
Conventional versus hypofractionated EBRT
The more convenient schedules of hypofractionated radiation therapy (using fewer fractions at higher doses per fraction) appear to yield similar outcomes to conventional schedules of radiation, at least with respect to the intermediate outcomes of DFS and failure-free survival (low levels of evidence not known to translate into health outcomes), and early data on OS rates. However, hypofractionated radiation may incur more toxicity than standard doses, depending on the schedules used.[71]
Evidence (conventional vs. hypofractionated EBRT):
- In a small randomized trial, primarily from one treatment center, conventional hypofractionation was not found to be superior to conventional fractionation.[72] In the trial, 303 assessable men were randomly assigned to receive IMRT for a total of 76 Gy in 38 fractions at 2.0 Gy per fraction (conventional IMRT [CIMRT]) versus IMRT for a total of 70.2 Gy in 26 fractions at 2.7 per fraction (hypofractionated IMRT [HIMRT]).
- The primary end point was biochemical or clinical disease failure (BCDF). The 5-year BCDF rates in the two arms were 21.4% for the CIMRT arm (95% CI, 14.8%–28.7%) and 23.3% for the HIMRT arm (95% CI, 16.4%–31.0%; P = .75).
- Likewise, there were no statistically significant differences in the secondary end points of overall mortality, prostate–cancer-specific mortality, prostate local failure, or distant failure, despite low mortality rates, and the trial was underpowered for mortality end points.[72][Level of evidence B1]
- The much larger, multicenter CHHiP trial (NCT00392535) evaluated conventional or hypofractionated high-dose intensity-modulated radiotherapy in 3,216 men with prostate cancer. The men had stages T1b–T3a, N0, M0 cancer and an estimated risk of seminal vesicle involvement of less than 30% and were randomly assigned in a 1:1:1 ratio to receive either 74 Gy in 37 fractions (the conventional-fraction arm), 60 Gy in 20 fractions, or 57 Gy in 19 fractions.[73,74] The trial was designed as a noninferiority study.
- The primary end point of biochemical or clinical treatment failure was reported after a median follow-up of 62.4 months. The 5-year failure-free survival rates were 88.3% (conventional, 74 Gy group), 90.6% (60 Gy group), and 85.9% (57 Gy group). The 60 Gy hypofractionated group fulfilled noninferiority criteria compared with conventional 74 Gy fractionation, but the 57 Gy group did not.[74][Level of evidence B1]
- Overall mortality rates were very similar in the three groups: 9%, 7%, and 8%.[74][Level of evidence A1]
- A QOL substudy was conducted with 2,100 participants and showed nearly identical patient-reported outcomes in each of the three arms at 2 years after study entry (median follow-up, 50 months).[73][Level of evidence A3]
- The primary patient-reported outcome was bowel bother. Frequency of moderate bother was 5%, 6%, and 5% in the three study groups. Severe bother was reported in less than 1% of men in each study group.
- Likewise, there were no differences in any of the secondary outcomes, which included overall QOL, overall urinary bother, or overall sexual bother.
- The multicenter, randomized, phase III HYPRO trial (ISRCTN85138529) enrolled 820 men with intermediate- or high-risk prostate cancer (stages T1b–T4, NX–0, MX–0). The men were randomly assigned to receive either conventional radiation therapy (78 Gy in 39 fractions over 8 weeks) or hypofractionated radiation therapy (64.6 Gy in 19 fractions over 6.5 weeks) in a noninferiority design for hypofractionation.[75,76] Median follow-up was 60 months.
- The primary end point, 5-year relapse-free survival, was similar in the two study arms: 80.5% (95% CI, 75.7%–84.4%) with hypofractionation versus 77.1% (95% CI, 71.9%–81.5%), with conventional fractionation (HR, 0.86; 95% CI, 0.63–1.16; P = .36).[76][Level of evidence B1] The overall 5-year survival rate in the two arms was also similar: 86.2% (95% CI, 82.3%–89.4%) with hypofractionation versus 85.9% (95% CI, 81.8%–89.2%) with conventional fractionation (HR, 1.02; 95% CI, 0.71–1.46; P = .92).[76][Level of evidence A1]
- With respect to toxicity (key end points of genitourinary [GU] or gastrointestinal [GI] grade 2 or higher toxicities at 3 years), noninferiority for hypofractionated radiation therapy could not be established after a median follow-up of 5 years: cumulative GU toxicity of 41.3% with hypofractionated radiation therapy versus 39% with conventional radiation therapy doses (HR, 1.16; 90% CI, 0.98–1.38); GI toxicity of 21.9% versus 17.7% (HR, 1.19; 90% CI, 0.93–1.52).
- Cumulative GU grade 3 or higher toxicity was more common in the hypofractionation group: 19.0% versus 12.9% (P = .02).
- Stool frequency (≥6 qd) was higher in the hypofractionation group: 7% versus 3% (P = .034).
- In a substudy of 322 men who had a baseline assessment and at least one follow-up assessment, and either no or short-term androgen therapy, erectile dysfunction was similar between the two study arms during 3 years of follow-up.[77]
- The RTOG reported a noninferiority trial of 1,115 men with low-risk prostate cancer (T1b–T2c) who were randomly assigned to receive hypofractionated radiation therapy (70 Gy in 28 fractions over 5.6 weeks) versus conventional radiation therapy doses (73.8 Gy in 41 fractions over 8.2 weeks).[78]
- After a median follow-up of 5.8 years, the hypofractionated radiation therapy arm met the prospective noninferiority criterion with respect to DFS: 86.3% with hypofractionated radiation therapy versus 85.3% with conventional radiation therapy doses (consistent with HR, <1.52; P < .001 for the hypothesis of noninferiority).[78][Level of evidence B1]
- There were 49 deaths in the hypofractionated radiation therapy arm and 51 deaths in the conventional radiation therapy doses arm (HR for OS, 0.95; conventional radiation therapy doses vs. hypofractionated radiation therapy; 95% CI, 0.64–1.41).
- However, late GI grade 2 or higher toxicity was worse in the hypofractionated radiation therapy arm: 22.4% versus 14.0% (P = .002); there was also a trend toward worse late GU grade 2 or higher toxicity: 29.7% versus 22.8% (P = .06).
- In a multicenter trial (NCT00304759), 1,206 men with intermediate-risk prostate cancer (T1–2a Gleason score ≤6, PSA 10.1–20 ng/mL; T2b–2c Gleason ≤6, PSA ≤20 ng/mL; or T1–2 Gleason = 7, PSA ≤20 ng/mL) were randomly assigned in a noninferiority trial design to receive conventional radiation therapy (78 Gy in 39 fractions) versus hypofractionated radiation therapy (60 Gy over 20 fractions).[79]
- After a median follow-up of 6 years (maximum 10 years), the primary end point of biochemical clinical failure (87%, PSA failure) was nearly identical with each radiation therapy schedule (85% in both arms; [DFS, 95% CI, 82%–88%]; HR, 0.96; 90% CI, 0.77–1.20).[79][Level of evidence B1]
- The trial was severely underpowered to detect any differences in overall or prostate-specific mortality. Only 12 deaths in the conventional radiation therapy arm and 10 deaths in the hypofractionated radiation therapy arm were from prostate cancer. Only 14% of all deaths were attributed to prostate cancer.
- Short- and long-term genitourinary and gastrointestinal toxicities were similar in both study groups.
Brachytherapy
Patients undergoing brachytherapy are often selected for favorable characteristics that include the following:
- Low Gleason score.
- Low PSA level.
- Stage T1 to T2 tumors.
More information and further study are required to better define the effects of modern interstitial brachytherapy on disease control and QOL and to determine the contribution of favorable patient selection to outcomes.[80][Level of evidence C3]
Information about ongoing clinical trials is available from the NCI website.
Radiopharmaceutical therapy
Alpha emitter radiation
Radium Ra 223 (223Ra) emits alpha particles (i.e., two protons and two neutrons bound together, identical to a helium nucleus) with a half-life of 11.4 days. It is administered intravenously and selectively taken up by newly formed bone stroma. The high-energy alpha particles have a short range of less than 100 mcM. 223Ra improved OS in patients with prostate cancer metastatic to bone. In a double-blind, randomized, controlled trial, 921 men with symptomatic castration-resistant prostate cancer, two or more metastases, and no known visceral metastases were randomly assigned in a 2:1 ratio to 223Ra versus placebo. 223Ra statistically significantly improved OS (median 14.9 months vs. 11.3 months), rate of symptomatic skeletal events (33% vs. 38%), and spinal cord compression (4% vs. 7%).[81,82][Level of evidence A1] With administration at a dose of 50kBq per kg body weight every 4 weeks for six injections, the side effects were similar to those of a placebo.
Complications of radiation therapy
Definitive EBRT can result in acute cystitis, proctitis, and enteritis.[20,42,49,83,84,85] These conditions are generally reversible but may be chronic and rarely require surgical intervention.[85]
A cross-sectional survey of patients with prostate cancer who had been treated in a managed care setting by radical prostatectomy, radiation therapy, or watchful waiting and active surveillance showed substantial sexual and urinary dysfunction in the radiation therapy group.[46]
Radiation is also carcinogenic.[86,87,88] EBRT for prostate cancer is associated with an increased risk of bladder and gastrointestinal cancer. Brachytherapy is associated with an increased risk of bladder cancer.
Reducing complications
Potency, in most cases, is preserved with radiation therapy in the short term but appears to diminish over time.[85] Sildenafil citrate may be effective in the management of sexual dysfunction after radiation therapy in some men.
Evidence (reducing complications):
- In a completed, randomized, placebo-controlled, crossover design study (RTOG-0215 [NCT00057759]) of 60 men who had undergone radiation therapy for clinically localized prostate cancer, and who reported erectile dysfunction that began after their radiation therapy, 55% reported successful intercourse after sildenafil versus 18% after placebo (P < .001).[89][Level of evidence A3]
- A randomized trial (RTOG-0831 [NCT00931528]) of 121 men with intact erectile function compared daily preventive tadalafil (5 mg PO qd) with placebo for 24 weeks beginning at the start of either EBRT or brachytherapy.[90][Level of evidence A3]
- There were no statistically significant differences in spontaneous erectile function (the primary end point) or any other measures of sexual function.
Morbidity may be reduced with the employment of sophisticated radiation therapy techniques—such as the use of linear accelerators—and careful simulation and treatment planning.[91,92]
Evidence (3D conformal vs. conventional radiation therapy):
- The side effects of similar doses of 3D conformal radiation therapy and conventional radiation therapy (total dose, 60–64 Gy) have been compared in a randomized nonblinded study.[92][Level of evidence A3]
- No differences were observed in acute morbidity, and late side effects serious enough to require hospitalization were infrequent with both techniques; however, the cumulative incidence of mild or greater proctitis was lower in the conformal radiation arm than in the standard therapy arm (37% vs. 56%; P = .004). Urinary symptoms were similar in the two treatment groups, as were local tumor control and OS rates at 5 years of follow-up.
Radiation therapy can be delivered after an extraperitoneal lymph node dissection without an increase in complications if careful attention is paid to radiation technique. The treatment field should not include the area that contained the dissected pelvic nodes. Previous TURP is associated with an increased risk of stricture above that seen with radiation therapy alone, but, if radiation therapy is delayed 4 to 6 weeks after the TURP, the risk of stricture is lower.[93,94,95] Pretreatment TURP to relieve obstructive symptoms has been associated with tumor dissemination; however, multivariable analysis in pathologically staged cases indicates that this may be due to a worse underlying prognosis of the cases that require TURP rather than the result of the procedure itself.[96]
Comparison of complications from radiation therapy and from radical prostatectomy
In general, radical prostatectomy is associated with a higher rate of urinary incontinence and early sexual impotence but a lower rate of stool incontinence and rectal injury. However, over time, the differences in sexual impotence diminish because the risk rises with time since radiation. Many side effects of definitive local therapy for prostate cancer persist well beyond a decade after therapy, and urinary problems in addition to sexual impotence may worsen with age.[97]
Evidence (complications of radical prostatectomy vs. radiation therapy):
- A population-based survey of Medicare recipients who had received radiation therapy as primary treatment for prostate cancer (similar in design to the survey of Medicare patients who underwent radical prostatectomy,[44] described above) has been reported, showing substantial differences in posttreatment morbidity profiles between surgery and radiation therapy.[98]
- Although the men who had undergone radiation therapy were older at the time of initial therapy, they were less likely to report the need for pads or clamps to control urinary wetness (7% vs. >30%).
- A larger proportion of patients treated with radiation therapy before surgery reported the ability to have an erection sufficient for intercourse in the month before the survey (men <70 years, 33% who received radiation therapy vs. 11% who underwent surgery alone; men ≥70 years, 27% who received radiation therapy vs. 12% who underwent surgery alone).
- Men receiving radiation therapy, however, were more likely to report problems with bowel function, especially frequent bowel movements (10% vs. 3%).
- As in the results of the surgical patient survey, about 24% of patients who received radiation reported additional subsequent treatment for known or suspected cancer persistence or recurrence within 3 years of primary therapy.
- A prospective, community-based cohort study of men aged 55 to 74 years treated with radical prostatectomy (n = 1,156) or EBRT (n = 435) attempted to compare the acute and chronic complications of the two treatment strategies after adjusting for baseline differences in patient characteristics and underlying health.[99]
- Regarding acute treatment-related morbidity, radical prostatectomy was associated with higher rates of cardiopulmonary complications (5.5% vs. 1.9%) and the need for treatment of urinary strictures (17.4% vs. 7.2%). Radiation therapy was associated with more acute rectal proctitis (18.7% vs. 1.6%).
- With regard to chronic treatment-related morbidity, radical prostatectomy was associated with more urinary incontinence (9.6% vs. 3.5%) and impotence (80% vs. 62%). Radiation therapy was associated with slightly greater declines in bowel function.
Hormonal Therapy and Its Complications
Several different hormonal approaches are used in the management of various stages of prostate cancer.
These approaches include the following:
- Abiraterone acetate (added to androgen deprivation therapy [ADT]).
- Bilateral orchiectomy.
- Estrogen therapy.
- Luteinizing hormone-releasing hormone (LH-RH) agonist therapy.
- Antiandrogen therapy.
- ADT.
- Antiadrenal therapy.
- Ketoconazole.
- Aminoglutethimide.
Abiraterone acetate
Abiraterone acetate has been shown to improve OS when added to ADT in men with advanced prostate cancer who have castration-sensitive disease. Abiraterone acetate is generally well-tolerated; however, it is associated with an increase in the mineralocorticoid effects of grade 3 or 4 hypertension and hypokalemia compared with ADT alone.[100] It may also be associated with a small increase in respiratory disorders.[101]
Bilateral orchiectomy
Benefits of bilateral orchiectomy include the following:[42]
- Ease of the procedure.
- Compliance.
- Immediacy in lowering testosterone levels.
- Low cost relative to the other forms of ADT.
Disadvantages of bilateral orchiectomy include the following:[42,102]
- Psychological effects.
- Loss of libido.
- Less reversible impotence.
- Hot flashes.
- Osteoporosis.[102]
Bilateral orchiectomy has also been associated with an elevated risk of coronary heart disease and myocardial infarction.[103,104,105,106]
For more information, see Hot Flashes and Night Sweats.
Estrogen therapy
Estrogens at a dose of 3 mg qd of diethylstilbestrol (DES) will achieve castrate levels of testosterone. Like orchiectomy, estrogens may cause loss of libido and impotence. Estrogens also cause gynecomastia, and prophylactic low-dose radiation therapy to the breasts is given to prevent this complication.
DES is no longer manufactured or marketed in the United States and is seldom used today because of the risk of serious side effects, including myocardial infarction, cerebrovascular accidents, and pulmonary embolism.
Luteinizing hormone-releasing hormone (LH-RH) agonist therapy
LH-RH agonists, such as leuprolide, goserelin, and buserelin, lower testosterone to castrate levels. Like orchiectomy and estrogens, LH-RH agonists cause impotence, hot flashes, and loss of libido. Tumor flare reactions may occur transiently but can be prevented by antiandrogens or short-term estrogens at a low dose for several weeks.
There is some evidence that LH-RH agonists are associated with increased risk of cardiovascular morbidity or mortality, although the results are conflicting.[103,104,105,106,107]
Evidence (LH-RH agonists and cardiovascular disease):
- In a population-based study within the Department of Veterans Affairs' system, LH-RH agonists were associated with an increased risk of diabetes as well as cardiovascular disease, including coronary heart disease, myocardial infarction, sudden death, and stroke.[103,104,105]
- A systematic evidence review and meta-analysis of eight trials (4,141 patients) of men with nonmetastatic prostate cancer who were randomly assigned to receive or not to receive LH-RH agonists found no difference in cardiovascular death rates (11.0% vs. 11.2%; RRdeath, 0.93; 95% CI, 0.79–1.10; P = .41).[108] Median follow-up in those studies was 7.6 to 13.2 years. No excess risk of LH-RH agonists was found regardless of treatment duration or patient age (median age of <70 years or ≥70 years).
Antiandrogen therapy
Antiandrogen agents used in the treatment of prostate cancer include flutamide and bicalutamide. A systematic evidence review compared nonsteroidal antiandrogen monotherapy with surgical or medical castration from 11 randomized trials in 3,060 men with locally advanced, metastatic, or recurrent disease after local therapy.[109] Use of nonsteroidal antiandrogens as monotherapy decreased OS and increased the rate of clinical progression and treatment failure.[109][Level of evidence A1]
The pure antiandrogen, flutamide, may cause diarrhea, breast tenderness, and nausea. Case reports show fatal and nonfatal liver toxic effects.[110] For more information, see Gastrointestinal Complications.
Bicalutamide may cause nausea, breast tenderness, hot flashes, loss of libido, and impotence.[111] For more information, see Nausea and Vomiting Related to Cancer Treatment and Hot Flashes and Night Sweats.
The steroidal antiandrogen, megestrol acetate, suppresses androgen production incompletely and is generally not used as initial therapy.
Additional studies that evaluate the effects of various hormone therapies on QOL are required.[112]
ADT
A national Medicare survey of men who had undergone radical prostatectomy for prostate cancer and either had or had not undergone androgen depletion (either medically or surgically induced) showed a decrease with androgen depletion in all seven health-related QOL measures, including the following:[113][Level of evidence C1]
- Impact of cancer and treatment.
- Concern regarding body image.
- Mental health.
- General health.
- Activity.
- Worries about cancer and dying.
- Energy.
ADT can cause osteoporosis and bone fractures. In a population-based sample of 50,613 Medicare patients aged 66 years or older followed for a median of 5.1 years, men who had been treated with either a gonadotropin-releasing hormone (GnRH) or orchiectomy had a 19.4% bone fracture rate compared with 12.6% in men who had not received hormone deprivation therapy. The effect was similar in men whether or not they had metastatic bone disease.[114]
The use of ADT may be associated with complaints of penile shortening, although the data are very limited.[60] In a registry study of men with rising PSA after initial treatment of clinically localized prostate cancer treated with radiation therapy plus ADT, 6 of 225 men (2.7%) complained of reduced penile size. Of the 213 men treated with radiation therapy but no ADT, none complained of changes in penile size. However, the data were based upon physician reporting of patients' complaints rather than direct patient questioning or before-and-after measurement of penile length. Also, the study sample was restricted to patients with known or suspected tumor recurrence, making generalization difficult.
Placebo-controlled, randomized trials have shown that treatment of bone loss with bisphosphonates decreases the risk of bone fracture in men receiving ADT for prostate cancer (RR, 0.80 in a meta-analysis of 15 trials; 95% CI, 0.69–0.94). In the meta-analysis, zoledronate appeared to have the largest effect.[115]
The use of ADT has also been associated with an increased risk of colorectal cancer.
Evidence (increased risk of colorectal cancer):
- Using the Surveillance, Epidemiology, and End Results (SEER) Medicare database, investigators assessed the risk of subsequent colorectal cancer in 107,859 men aged 67 years and older after an initial diagnosis of prostate cancer.[116]
- The rates of colorectal cancer per 1,000 person-years were 6.3 (95% CI, 5.3–7.5) in men who had orchiectomy, 4.4 (95% CI, 4.0–4.9) in men treated with GnRH agonists, and 3.7 (95% CI, 3.5–3.9) in men who had no androgen deprivation.
- In men treated with GnRH agonists, the risk increased with increasing duration of treatment (P for trend = .01).
Antiadrenal therapy
Antiadrenal agents used in the treatment of prostate cancer include ketoconazole and aminoglutethimide. Long-term use of ketoconazole can result in impotence, pruritus, nail changes, and adrenal insufficiency. Aminoglutethimide commonly causes sedation and skin rashes. For more information, see Pruritus.
Cryosurgery
Cryosurgery, or cryotherapy, is under evaluation for the treatment of localized prostate cancer. It is a surgical technique that involves destruction of prostate cancer cells by intermittent freezing of the prostate with cryoprobes, followed by thawing.[117][Level of evidence C1]; [118,119][Level of evidence C3] There is limited evidence regarding its efficacy and safety compared with standard prostatectomy and radiation therapy, and the technique is evolving in an attempt to reduce local toxicity and normal tissue damage. The quality of evidence on efficacy is low, currently limited to case series of relatively small size, short follow-up, and surrogate outcomes of efficacy.[120]
Serious toxic effects associated with cryosurgery include bladder outlet injury, urinary incontinence, sexual impotence, and rectal injury. Impotence is common, ranging from about 47% to 100%.
The frequency of other side effects and the probability of cancer control at 5 years' follow-up have varied among reporting centers, and series are small compared with surgery and radiation therapy.[118,119] Other major complications include urethral sloughing, urinary fistula or stricture, and bladder neck obstruction.[120]
Proton-Beam Therapy
There is interest in the use of proton-beam therapy for the treatment of prostate cancer. Although the dose distribution of this form of charged-particle radiation could theoretically improve the therapeutic ratio of prostate radiation, allowing for an increase in dose to the tumor without a substantial increase in side effects, no randomized controlled trials have been reported that compare its efficacy and toxicity with those of other forms of radiation therapy.
Photodynamic Therapy
Vascular-targeted photodynamic therapy using a photosensitizing agent has been tested in men with low-risk prostate cancer.[121]
Neoadjuvant Hormonal Therapy
The role of neoadjuvant hormonal therapy is not established.[26,27]
Bicalutamide
Bicalutamide has not been shown to improve OS in patients with localized or locally advanced prostate cancer.
Evidence (bicalutamide):
- The Early Prostate Cancer program is a large, randomized, placebo-controlled, international trial that compared bicalutamide (150 mg PO qd) plus standard care (radical prostatectomy, radiation therapy, or watchful waiting, depending on local custom) with standard care alone for men with nonmetastatic localized or locally advanced prostate cancer (T1–2, N0, and NX; T3–4, any N; or any T, N+). Less than 2% of the 8,113 men had known nodal disease.[122][Level of evidence A1]
- At a median follow-up of 7.4 years, there was no difference in OS between the bicalutamide and placebo groups (about 76% in both arms [HR, 0.99; CI, 95%, 0.91–1.09; P = .89]).
Information about ongoing clinical trials is available from the NCI website.
References:
-
Barocas DA, Alvarez J, Resnick MJ, et al.: Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 317 (11): 1126-1140, 2017.
-
Chen RC, Basak R, Meyer AM, et al.: Association Between Choice of Radical Prostatectomy, External Beam Radiotherapy, Brachytherapy, or Active Surveillance and Patient-Reported Quality of Life Among Men With Localized Prostate Cancer. JAMA 317 (11): 1141-1150, 2017.
-
Chodak GW, Thisted RA, Gerber GS, et al.: Results of conservative management of clinically localized prostate cancer. N Engl J Med 330 (4): 242-8, 1994.
-
Whitmore WF: Expectant management of clinically localized prostatic cancer. Semin Oncol 21 (5): 560-8, 1994.
-
Wilt TJ, Brawer MK, Jones KM, et al.: Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 367 (3): 203-13, 2012.
-
Shappley WV, Kenfield SA, Kasperzyk JL, et al.: Prospective study of determinants and outcomes of deferred treatment or watchful waiting among men with prostate cancer in a nationwide cohort. J Clin Oncol 27 (30): 4980-5, 2009.
-
Klotz L: Active surveillance with selective delayed intervention: using natural history to guide treatment in good risk prostate cancer. J Urol 172 (5 Pt 2): S48-50; discussion S50-1, 2004.
-
Carter HB, Walsh PC, Landis P, et al.: Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol 167 (3): 1231-4, 2002.
-
Klotz L, Vesprini D, Sethukavalan P, et al.: Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 33 (3): 272-7, 2015.
-
Johansson JE, Holmberg L, Johansson S, et al.: Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA 277 (6): 467-71, 1997.
-
Johansson JE, Andrén O, Andersson SO, et al.: Natural history of early, localized prostate cancer. JAMA 291 (22): 2713-9, 2004.
-
Waaler G, Stenwig AE: Prognosis of localised prostatic cancer managed by "watch and wait" policy. Br J Urol 72 (2): 214-9, 1993.
-
Lu-Yao GL, Albertsen PC, Moore DF, et al.: Outcomes of localized prostate cancer following conservative management. JAMA 302 (11): 1202-9, 2009.
-
Stattin P, Holmberg E, Johansson JE, et al.: Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst 102 (13): 950-8, 2010.
-
Holmström B, Holmberg E, Egevad L, et al.: Outcome of primary versus deferred radical prostatectomy in the National Prostate Cancer Register of Sweden Follow-Up Study. J Urol 184 (4): 1322-7, 2010.
-
Barry MJ, Albertsen PC, Bagshaw MA, et al.: Outcomes for men with clinically nonmetastatic prostate carcinoma managed with radical prostactectomy, external beam radiotherapy, or expectant management: a retrospective analysis. Cancer 91 (12): 2302-14, 2001.
-
Lu-Yao GL, Yao SL: Population-based study of long-term survival in patients with clinically localised prostate cancer. Lancet 349 (9056): 906-10, 1997.
-
van den Bergh RC, Roemeling S, Roobol MJ, et al.: Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol 55 (1): 1-8, 2009.
-
Hamdy FC, Donovan JL, Lane JA, et al.: 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 375 (15): 1415-1424, 2016.
-
Catalona WJ, Bigg SW: Nerve-sparing radical prostatectomy: evaluation of results after 250 patients. J Urol 143 (3): 538-43; discussion 544, 1990.
-
Corral DA, Bahnson RR: Survival of men with clinically localized prostate cancer detected in the eighth decade of life. J Urol 151 (5): 1326-9, 1994.
-
Zincke H, Bergstralh EJ, Blute ML, et al.: Radical prostatectomy for clinically localized prostate cancer: long-term results of 1,143 patients from a single institution. J Clin Oncol 12 (11): 2254-63, 1994.
-
Schuessler WW, Vancaillie TG, Reich H, et al.: Transperitoneal endosurgical lymphadenectomy in patients with localized prostate cancer. J Urol 145 (5): 988-91, 1991.
-
Coughlin GD, Yaxley JW, Chambers SK, et al.: Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol 19 (8): 1051-1060, 2018.
-
Fournier GR, Narayan P: Re-evaluation of the need for pelvic lymphadenectomy in low grade prostate cancer. Br J Urol 72 (4): 484-8, 1993.
-
Witjes WP, Schulman CC, Debruyne FM: Preliminary results of a prospective randomized study comparing radical prostatectomy versus radical prostatectomy associated with neoadjuvant hormonal combination therapy in T2-3 N0 M0 prostatic carcinoma. The European Study Group on Neoadjuvant Treatment of Prostate Cancer. Urology 49 (3A Suppl): 65-9, 1997.
-
Fair WR, Cookson MS, Stroumbakis N, et al.: The indications, rationale, and results of neoadjuvant androgen deprivation in the treatment of prostatic cancer: Memorial Sloan-Kettering Cancer Center results. Urology 49 (3A Suppl): 46-55, 1997.
-
Adolfsson J, Rönström L, Löwhagen T, et al.: Deferred treatment of clinically localized low grade prostate cancer: the experience from a prospective series at the Karolinska Hospital. J Urol 152 (5 Pt 2): 1757-60, 1994.
-
Grossfeld GD, Chang JJ, Broering JM, et al.: Impact of positive surgical margins on prostate cancer recurrence and the use of secondary cancer treatment: data from the CaPSURE database. J Urol 163 (4): 1171-7; quiz 1295, 2000.
-
Wasson JH, Cushman CC, Bruskewitz RC, et al.: A structured literature review of treatment for localized prostate cancer. Prostate Disease Patient Outcome Research Team. Arch Fam Med 2 (5): 487-93, 1993.
-
Adolfsson J, Steineck G, Whitmore WF: Recent results of management of palpable clinically localized prostate cancer. Cancer 72 (2): 310-22, 1993.
-
Austenfeld MS, Thompson IM, Middleton RG: Meta-analysis of the literature: guideline development for prostate cancer treatment. American Urological Association Prostate Cancer Guideline Panel. J Urol 152 (5 Pt 2): 1866-9, 1994.
-
Holmberg L, Bill-Axelson A, Helgesen F, et al.: A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med 347 (11): 781-9, 2002.
-
Bill-Axelson A, Holmberg L, Ruutu M, et al.: Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 352 (19): 1977-84, 2005.
-
Bill-Axelson A, Holmberg L, Garmo H, et al.: Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 370 (10): 932-42, 2014.
-
Wilt TJ: The Prostate Cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy with watchful waiting for men with clinically localized prostate cancer. J Natl Cancer Inst Monogr 2012 (45): 184-90, 2012.
-
Wilt TJ, Jones KM, Barry MJ, et al.: Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl J Med 377 (2): 132-142, 2017.
-
Donovan JL, Hamdy FC, Lane JA, et al.: Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 375 (15): 1425-1437, 2016.
-
Yao SL, Lu-Yao G: Population-based study of relationships between hospital volume of prostatectomies, patient outcomes, and length of hospital stay. J Natl Cancer Inst 91 (22): 1950-6, 1999.
-
Lu-Yao GL, McLerran D, Wasson J, et al.: An assessment of radical prostatectomy. Time trends, geographic variation, and outcomes. The Prostate Patient Outcomes Research Team. JAMA 269 (20): 2633-6, 1993.
-
Alibhai SM, Leach M, Tomlinson G, et al.: 30-day mortality and major complications after radical prostatectomy: influence of age and comorbidity. J Natl Cancer Inst 97 (20): 1525-32, 2005.
-
Sanda MG, Dunn RL, Michalski J, et al.: Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 358 (12): 1250-61, 2008.
-
Catalona WJ, Basler JW: Return of erections and urinary continence following nerve sparing radical retropubic prostatectomy. J Urol 150 (3): 905-7, 1993.
-
Fowler FJ, Barry MJ, Lu-Yao G, et al.: Patient-reported complications and follow-up treatment after radical prostatectomy. The National Medicare Experience: 1988-1990 (updated June 1993). Urology 42 (6): 622-9, 1993.
-
Potosky AL, Davis WW, Hoffman RM, et al.: Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst 96 (18): 1358-67, 2004.
-
Litwin MS, Hays RD, Fink A, et al.: Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 273 (2): 129-35, 1995.
-
Jønler M, Messing EM, Rhodes PR, et al.: Sequelae of radical prostatectomy. Br J Urol 74 (3): 352-8, 1994.
-
Geary ES, Dendinger TE, Freiha FS, et al.: Nerve sparing radical prostatectomy: a different view. J Urol 154 (1): 145-9, 1995.
-
Lim AJ, Brandon AH, Fiedler J, et al.: Quality of life: radical prostatectomy versus radiation therapy for prostate cancer. J Urol 154 (4): 1420-5, 1995.
-
Savoie M, Kim SS, Soloway MS: A prospective study measuring penile length in men treated with radical prostatectomy for prostate cancer. J Urol 169 (4): 1462-4, 2003.
-
Gontero P, Galzerano M, Bartoletti R, et al.: New insights into the pathogenesis of penile shortening after radical prostatectomy and the role of postoperative sexual function. J Urol 178 (2): 602-7, 2007.
-
McCullough A: Penile change following radical prostatectomy: size, smooth muscle atrophy, and curve. Curr Urol Rep 9 (6): 492-9, 2008.
-
Sun M, Lughezzani G, Alasker A, et al.: Comparative study of inguinal hernia repair after radical prostatectomy, prostate biopsy, transurethral resection of the prostate or pelvic lymph node dissection. J Urol 183 (3): 970-5, 2010.
-
Sekita N, Suzuki H, Kamijima S, et al.: Incidence of inguinal hernia after prostate surgery: open radical retropubic prostatectomy versus open simple prostatectomy versus transurethral resection of the prostate. Int J Urol 16 (1): 110-3, 2009.
-
Lughezzani G, Sun M, Perrotte P, et al.: Comparative study of inguinal hernia repair rates after radical prostatectomy or external beam radiotherapy. Int J Radiat Oncol Biol Phys 78 (5): 1307-13, 2010.
-
Lodding P, Bergdahl C, Nyberg M, et al.: Inguinal hernia after radical retropubic prostatectomy for prostate cancer: a study of incidence and risk factors in comparison to no operation and lymphadenectomy. J Urol 166 (3): 964-7, 2001.
-
Lepor H, Robbins D: Inguinal hernias in men undergoing open radical retropubic prostatectomy. Urology 70 (5): 961-4, 2007.
-
Bishoff JT, Motley G, Optenberg SA, et al.: Incidence of fecal and urinary incontinence following radical perineal and retropubic prostatectomy in a national population. J Urol 160 (2): 454-8, 1998.
-
Nossiter J, Sujenthiran A, Charman SC, et al.: Robot-assisted radical prostatectomy vs laparoscopic and open retropubic radical prostatectomy: functional outcomes 18 months after diagnosis from a national cohort study in England. Br J Cancer 118 (4): 489-494, 2018.
-
Parekh A, Chen MH, Hoffman KE, et al.: Reduced penile size and treatment regret in men with recurrent prostate cancer after surgery, radiotherapy plus androgen deprivation, or radiotherapy alone. Urology 81 (1): 130-4, 2013.
-
Kadono Y, Machioka K, Nakashima K, et al.: Changes in penile length after radical prostatectomy: investigation of the underlying anatomical mechanism. BJU Int 120 (2): 293-299, 2017.
-
Forman JD, Order SE, Zinreich ES, et al.: Carcinoma of the prostate in the elderly: the therapeutic ratio of definitive radiotherapy. J Urol 136 (6): 1238-41, 1986.
-
Duncan W, Warde P, Catton CN, et al.: Carcinoma of the prostate: results of radical radiotherapy (1970-1985) Int J Radiat Oncol Biol Phys 26 (2): 203-10, 1993.
-
Zietman AL, Coen JJ, Shipley WU, et al.: Radical radiation therapy in the management of prostatic adenocarcinoma: the initial prostate specific antigen value as a predictor of treatment outcome. J Urol 151 (3): 640-5, 1994.
-
Peeters ST, Heemsbergen WD, Koper PC, et al.: Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 24 (13): 1990-6, 2006.
-
Zietman AL, DeSilvio ML, Slater JD, et al.: Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 294 (10): 1233-9, 2005.
-
Pollack A, Zagars GK, Starkschall G, et al.: Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 53 (5): 1097-105, 2002.
-
Dearnaley DP, Jovic G, Syndikus I, et al.: Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 15 (4): 464-73, 2014.
-
Michalski JM, Moughan J, Purdy J, et al.: Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol 4 (6): e180039, 2018.
-
Asbell SO, Martz KL, Shin KH, et al.: Impact of surgical staging in evaluating the radiotherapeutic outcome in RTOG #77-06, a phase III study for T1BN0M0 (A2) and T2N0M0 (B) prostate carcinoma. Int J Radiat Oncol Biol Phys 40 (4): 769-82, 1998.
-
Yu JB: Hypofractionated Radiotherapy for Prostate Cancer: Further Evidence to Tip the Scales. J Clin Oncol 35 (17): 1867-1869, 2017.
-
Pollack A, Walker G, Horwitz EM, et al.: Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 31 (31): 3860-8, 2013.
-
Wilkins A, Mossop H, Syndikus I, et al.: Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 16 (16): 1605-16, 2015.
-
Dearnaley D, Syndikus I, Mossop H, et al.: Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 17 (8): 1047-60, 2016.
-
Aluwini S, Pos F, Schimmel E, et al.: Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol 17 (4): 464-74, 2016.
-
Incrocci L, Wortel RC, Alemayehu WG, et al.: Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 17 (8): 1061-9, 2016.
-
Wortel RC, Pos FJ, Heemsbergen WD, et al.: Sexual Function After Hypofractionated Versus Conventionally Fractionated Radiotherapy for Prostate Cancer: Results From the Randomized Phase III HYPRO Trial. J Sex Med 13 (11): 1695-1703, 2016.
-
Lee WR, Dignam JJ, Amin MB, et al.: Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol 34 (20): 2325-32, 2016.
-
Catton CN, Lukka H, Gu CS, et al.: Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol 35 (17): 1884-1890, 2017.
-
Ragde H, Blasko JC, Grimm PD, et al.: Interstitial iodine-125 radiation without adjuvant therapy in the treatment of clinically localized prostate carcinoma. Cancer 80 (3): 442-53, 1997.
-
Parker C, Nilsson S, Heinrich D, et al.: Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369 (3): 213-23, 2013.
-
Sartor O, Coleman R, Nilsson S, et al.: Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 15 (7): 738-46, 2014.
-
Schellhammer PF, Jordan GH, el-Mahdi AM: Pelvic complications after interstitial and external beam irradiation of urologic and gynecologic malignancy. World J Surg 10 (2): 259-68, 1986.
-
Lee JY, Daignault-Newton S, Heath G, et al.: Multinational Prospective Study of Patient-Reported Outcomes After Prostate Radiation Therapy: Detailed Assessment of Rectal Bleeding. Int J Radiat Oncol Biol Phys 96 (4): 770-777, 2016.
-
Hamilton AS, Stanford JL, Gilliland FD, et al.: Health outcomes after external-beam radiation therapy for clinically localized prostate cancer: results from the Prostate Cancer Outcomes Study. J Clin Oncol 19 (9): 2517-26, 2001.
-
Nieder AM, Porter MP, Soloway MS: Radiation therapy for prostate cancer increases subsequent risk of bladder and rectal cancer: a population based cohort study. J Urol 180 (5): 2005-9; discussion 2009-10, 2008.
-
Abdel-Wahab M, Reis IM, Wu J, et al.: Second primary cancer risk of radiation therapy after radical prostatectomy for prostate cancer: an analysis of SEER data. Urology 74 (4): 866-71, 2009.
-
Nam RK, Cheung P, Herschorn S, et al.: Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. Lancet Oncol 15 (2): 223-31, 2014.
-
Incrocci L, Koper PC, Hop WC, et al.: Sildenafil citrate (Viagra) and erectile dysfunction following external beam radiotherapy for prostate cancer: a randomized, double-blind, placebo-controlled, cross-over study. Int J Radiat Oncol Biol Phys 51 (5): 1190-5, 2001.
-
Pisansky TM, Pugh SL, Greenberg RE, et al.: Tadalafil for prevention of erectile dysfunction after radiotherapy for prostate cancer: the Radiation Therapy Oncology Group [0831] randomized clinical trial. JAMA 311 (13): 1300-7, 2014.
-
Hanks GE, Hanlon AL, Schultheiss TE, et al.: Dose escalation with 3D conformal treatment: five year outcomes, treatment optimization, and future directions. Int J Radiat Oncol Biol Phys 41 (3): 501-10, 1998.
-
Dearnaley DP, Khoo VS, Norman AR, et al.: Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet 353 (9149): 267-72, 1999.
-
Greskovich FJ, Zagars GK, Sherman NE, et al.: Complications following external beam radiation therapy for prostate cancer: an analysis of patients treated with and without staging pelvic lymphadenectomy. J Urol 146 (3): 798-802, 1991.
-
Seymore CH, el-Mahdi AM, Schellhammer PF: The effect of prior transurethral resection of the prostate on post radiation urethral strictures and bladder neck contractures. Int J Radiat Oncol Biol Phys 12 (9): 1597-600, 1986.
-
Green N, Treible D, Wallack H, et al.: Prostate cancer--the impact of irradiation on urinary outlet obstruction. Br J Urol 70 (3): 310-3, 1992.
-
Zelefsky MJ, Whitmore WF, Leibel SA, et al.: Impact of transurethral resection on the long-term outcome of patients with prostatic carcinoma. J Urol 150 (6): 1860-4, 1993.
-
Jang JW, Drumm MR, Efstathiou JA, et al.: Long-term quality of life after definitive treatment for prostate cancer: patient-reported outcomes in the second posttreatment decade. Cancer Med 6 (7): 1827-1836, 2017.
-
Fowler FJ, Barry MJ, Lu-Yao G, et al.: Outcomes of external-beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three surveillance, epidemiology, and end results areas. J Clin Oncol 14 (8): 2258-65, 1996.
-
Potosky AL, Legler J, Albertsen PC, et al.: Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 92 (19): 1582-92, 2000.
-
Fizazi K, Tran N, Fein L, et al.: Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 377 (4): 352-360, 2017.
-
James ND, de Bono JS, Spears MR, et al.: Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 377 (4): 338-351, 2017.
-
Daniell HW: Osteoporosis after orchiectomy for prostate cancer. J Urol 157 (2): 439-44, 1997.
-
Keating NL, O'Malley AJ, Freedland SJ, et al.: Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 102 (1): 39-46, 2010.
-
Keating NL, O'Malley AJ, Smith MR: Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24 (27): 4448-56, 2006.
-
D'Amico AV, Denham JW, Crook J, et al.: Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol 25 (17): 2420-5, 2007.
-
O'Farrell S, Garmo H, Holmberg L, et al.: Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol 33 (11): 1243-51, 2015.
-
Levine GN, D'Amico AV, Berger P, et al.: Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin 60 (3): 194-201, 2010 May-Jun.
-
Nguyen PL, Je Y, Schutz FA, et al.: Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 306 (21): 2359-66, 2011.
-
Kunath F, Grobe HR, Rücker G, et al.: Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev (6): CD009266, 2014.
-
Wysowski DK, Freiman JP, Tourtelot JB, et al.: Fatal and nonfatal hepatotoxicity associated with flutamide. Ann Intern Med 118 (11): 860-4, 1993.
-
Soloway MS, Schellhammer PF, Smith JA, et al.: Bicalutamide in the treatment of advanced prostatic carcinoma: a phase II multicenter trial. Urology 47 (1A Suppl): 33-7; discussion 48-53, 1996.
-
Kirschenbaum A: Management of hormonal treatment effects. Cancer 75 (7 Suppl): 1983-86, 1995.
-
Fowler FJ, McNaughton Collins M, Walker Corkery E, et al.: The impact of androgen deprivation on quality of life after radical prostatectomy for prostate carcinoma. Cancer 95 (2): 287-95, 2002.
-
Shahinian VB, Kuo YF, Freeman JL, et al.: Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352 (2): 154-64, 2005.
-
Serpa Neto A, Tobias-Machado M, Esteves MA, et al.: Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 15 (1): 36-44, 2012.
-
Gillessen S, Templeton A, Marra G, et al.: Risk of colorectal cancer in men on long-term androgen deprivation therapy for prostate cancer. J Natl Cancer Inst 102 (23): 1760-70, 2010.
-
Robinson JW, Saliken JC, Donnelly BJ, et al.: Quality-of-life outcomes for men treated with cryosurgery for localized prostate carcinoma. Cancer 86 (9): 1793-801, 1999.
-
Donnelly BJ, Saliken JC, Ernst DS, et al.: Prospective trial of cryosurgical ablation of the prostate: five-year results. Urology 60 (4): 645-9, 2002.
-
Aus G, Pileblad E, Hugosson J: Cryosurgical ablation of the prostate: 5-year follow-up of a prospective study. Eur Urol 42 (2): 133-8, 2002.
-
Shelley M, Wilt TJ, Coles B, et al.: Cryotherapy for localised prostate cancer. Cochrane Database Syst Rev (3): CD005010, 2007.
-
Azzouzi AR, Vincendeau S, Barret E, et al.: Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 18 (2): 181-191, 2017.
-
McLeod DG, Iversen P, See WA, et al.: Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int 97 (2): 247-54, 2006.
Treatment of Stage I Prostate Cancer
Overview
Stage I prostate cancer is defined by the American Joint Committee on Cancer's TNM (tumor, node, metastasis) classification system:[1]
- cT1a–c, N0, M0, prostate-specific antigen (PSA) <10 ng/mL, Gleason ≤6.
- cT2a, N0, M0, PSA <10 ng/mL, Gleason ≤6.
- pT2, N0, M0, PSA <10 ng/mL, Gleason ≤6.
The frequency of clinically silent, nonmetastatic prostate cancer that can be found at autopsy greatly increases with age and may be as high as 50% to 60% in men aged 90 years and older. Undoubtedly, the incidental discovery of these occult cancers at prostatic surgery performed for other reasons accounts for the similar survival of men with stage I prostate cancer, compared with the normal male population, adjusted for age.
Many stage I cancers are well differentiated and only focally involve the gland (T1a, N0, M0); most require no treatment other than careful follow-up.[2]
In younger patients (aged 50–60 years) whose expected survival is long, treatment should be considered.[3] Radical prostatectomy, external-beam radiation therapy (EBRT), interstitial implantation of radioisotopes, and watchful waiting and active surveillance/active monitoring yield apparently similar survival rates in noncontrolled, selected series. The decision to treat should be made in the context of the patient's age, associated medical illnesses, and personal desires.[3]
Treatment Options for Stage I Prostate Cancer
Treatment options for patients with stage I prostate cancer include the following:
- Watchful waiting or active surveillance/active monitoring.
- Radical prostatectomy.
- External-beam radiation therapy (EBRT).
- Interstitial implantation of radioisotopes.
- High-intensity focused ultrasound therapy (under clinical evaluation).[4,5,6,7]
- Photodynamic therapy (under clinical evaluation).
Watchful waiting or active surveillance/active monitoring
Asymptomatic patients of advanced age or with concomitant illness may warrant consideration of careful observation without immediate active treatment.[8,9,10] Watch and wait, observation, expectant management, and active surveillance/active monitoring are terms indicating a strategy that does not employ immediate therapy with curative intent. For more information, see the Watchful Waiting or Active Surveillance/Active Monitoring section.
Evidence (observation with delayed hormonal therapy):
- In a retrospective pooled analysis, 828 men with clinically localized prostate cancer were managed by initial conservative therapy with subsequent hormonal therapy given at the time of symptomatic disease progression.
- This study showed that the patients with grade 1 or grade 2 tumors experienced a disease-specific survival of 87% at 10 years and that their overall survival (OS) closely approximated the expected survival among men of similar ages in the general population.[8]
Radical prostatectomy
Radical prostatectomy, usually with pelvic lymphadenectomy (with or without the nerve-sparing technique designed to preserve potency) is the most commonly applied therapy with curative intent.[11,12,13] Radical prostatectomy may be difficult after a transurethral resection of the prostate (TURP).
Because about 40% to 50% of men with clinically organ-confined disease are found to have pathological extension beyond the prostate capsule or surgical margins, the role of postprostatectomy adjuvant radiation therapy has been studied.
Consideration may also be given to postoperative radiation therapy (PORT) for patients who are found to have seminal vesicle invasion by tumor at the time of prostatectomy or who have a detectable level of PSA more than 3 weeks after surgery.[14,15,16] Because duration of follow-up in available studies is still relatively short, the value of PORT has not been determined; however, PORT does reduce local recurrence.[14] Careful treatment planning is necessary to avoid morbidity.
Evidence (radical prostatectomy followed by radiation therapy):
- In a randomized trial of 425 men with pathological T3, N0, and M0 disease, postsurgical EBRT (60–64 Gy to the prostatic fossa over 30–32 fractions) was compared with observation.[15][Level of evidence A1]
- The primary end point, metastasis-free survival, could be affected by serial PSA monitoring and resulting metastatic work-up for PSA increase. This could have biased the primary end point in favor of radiation therapy, which was associated with a lower rate of PSA rise. Nevertheless, metastasis-free survival was not statistically different between the two study arms (P = .06). After a median follow-up of about 10.6 years, the overall median survival was 14.7 years in the radiation therapy group versus 13.8 years in the observation group (P = .16).
- Although the OS rates were not statistically different, complication rates were substantially higher in the radiation therapy group: overall complications were 23.8% versus 11.9%, rectal complications were 3.3% versus 0%, and urethral stricture was 17.8% versus 9.5%.
- After a median follow-up of about 12.5 years, however, OS was better in the radiation therapy arm; hazard ratio (HR)death, 0.72 (95% confidence interval [CI], 0.55–0.96; P = .023). The 10-year estimated survival rates were 74% in the radiation therapy arm and 66% in the control arm. The 10-year estimated metastasis-free survivals were 73% and 65% (P = .016).[16][Level of evidence A1]
- Another randomized trial came to a different conclusion with respect to the effect of postoperative radiation therapy on OS.[17][Level of evidence A1] In the European Organisation for Research and Treatment of Cancer (EORTC) trial (EORTC-22911 [NCT00002511]), 1,005 men aged 75 years and younger with clinical T0 to T3 prostate cancer were randomly assigned after prostatectomy to receive PORT (60 Gy) or observation, with subsequent therapy delayed until the occurrence of either biochemical or clinical relapse. The recommended treatment for local recurrence was radiation.
- With a median follow-up of 10.6 years (up to 16.6 years), the biochemical progression-free survival (PFS) rates were higher in the observation study arm (60.6% vs. 41.1%; HR, 0.49; 95% CI, 0.41–0.59; P < .0001). Locoregional relapse rates were 8.4% versus 17.3% in favor of immediate radiation (HR, 0.45; 95% CI, 0.32–0.68; P < .0001).
- However, the large differences in biochemical relapse-free survival and local recurrence did not translate into an advantage in either distant metastasis (11.0% vs. 11.3%; HR, 0.99; 95% CI, 0.67–1.44; P = .94) or in OS (76.9% with immediate radiation vs. 80.7% with observation; HR, 1.18; 95% CI, 0.91–1.53; P = .2). Nor was there a difference in prostate– cancer-specific mortality (3.9% vs. 5.2%; HR, 0.78; 95% CI, 0.46–1.33; P = .34)
- The 10-year cumulative risk of severe (grade 3) late toxicity in the immediate radiation study group was 5.3% versus 2.5% in the observation group (P = .052). Late adverse effects of any grade were also higher in the immediate radiation group (70.8% vs. 59.7%; P = .001).
Radical prostatectomy has been compared with watchful waiting or active surveillance/active monitoring. For more information, see the Radical prostatectomy compared with other treatment options section.
Evidence (radical prostatectomy compared with watchful waiting):
- The Prostate Intervention Versus Observation Trial (PIVOT-1 or VA-CSP-407 [NCT00007644]) is a randomized trial conducted in the PSA screening era that directly compared radical prostatectomy with watchful waiting. From November 1994 through January 2002, 731 men aged 75 years or younger with localized prostate cancer (stage T1–2, NX, M0, with a blood PSA <50 ng/mL) and a life expectancy of at least 10 years were randomly assigned to radical prostatectomy versus watchful waiting.[18,19,20][Level of evidence A1]
- About 50% of the men had nonpalpable, screen-detected disease.
- After a median follow-up of 12.7 years (range up to about 19.5 years), the all-cause mortality was 61.3% versus 66.8% in the prostatectomy and watchful-waiting study arms, respectively, an absolute difference of 5.5 percentage points (95% CI -1.5 to 12.4) that was not statistically significant (HR, 0.84; 95% CI, 0.70–1.01). Prostate–cancer-specific mortality was 7.4% versus 11.4%, and it also was not statistically significant (HR, 0.63; 95% CI, 0.3–1.02).
- Although treatment for disease progression was given more frequently in the observation arm of the study, most of the treatment was for asymptomatic, local, or biochemical (PSA) progression.
- As expected, urinary incontinence and erectile/sexual dysfunction was more common in the prostatectomy group for at least 10 years of follow-up. Absolute differences in patient-reported use of absorbent urinary pads was greater in the surgery group by more than 30 percentage points at all time points for at least 10 years. Disease- or treatment-related limitations in activities of daily living were worse with surgery than with observation through 2 years, but then were similar in both study arms.
External-beam radiation therapy (EBRT)
EBRT is another treatment option used with curative intent.[21,22,23,24,25] Definitive radiation therapy should be delayed 4 to 6 weeks after TURP to reduce the incidence of stricture.[26] Adjuvant hormonal therapy should be considered for patients with bulky T2b to T2c tumors.[27,28]
Evidence (EBRT with or without adjuvant hormonal therapy):
- In the Radiation Therapy Oncology Group (RTOG) trial RTOG-7706, prophylactic radiation therapy to clinically or pathologically uninvolved pelvic lymph nodes did not appear to improve OS or prostate cancer-specific survival.[29][Level of evidence A1]
- The phase III randomized RTOG-9413 trial (NCT00769548) included 1,323 men with localized prostate cancer, an elevated PSA, and an estimated risk of lymph node involvement of 15%. Patients were randomly assigned to one of four treatment arms: whole-pelvic radiation therapy plus neoadjuvant and concurrent hormonal therapy; prostate-only radiation therapy plus neoadjuvant and concurrent hormonal therapy; whole-pelvic radiation therapy plus adjuvant hormonal therapy; or pelvic-only radiation therapy plus adjuvant hormonal therapy.[30]; [31][Level of evidence B1]
- Although RTOG-9413 showed increased PFS at 4 years for patients who had a 15% estimated risk of lymph node involvement and received whole-pelvic radiation therapy compared with prostate-only radiation therapy, OS and PSA failure rates were not significantly different.
- In a randomized trial, 875 men with locally advanced nonmetastatic prostate cancer (T1b–T2 moderately or poorly differentiated tumors; T3 tumors of any grade) were randomly assigned to receive 3 months of a luteinizing hormone-releasing hormone agonist plus long-term flutamide (250 mg PO tid) with or without EBRT.[28][Level of evidence A1]
- Nineteen percent of the men had tumor stage T2, and 78% of the men had T3. At 10 years, both overall mortality (29.6% vs. 39.4%; 95% CI for the difference, 0.8%–18.8%) and the prostate–cancer-specific mortality (11.9% vs. 23.9%; 95% CI for the difference, 4.9%–19.1%) favored combined hormonal and radiation therapy.
- Although flutamide might not be considered a standard hormonal monotherapy in the setting of T2 or T3 tumors, radiation therapy provided a disease-free survival or tumor-specific survival advantage even though this monotherapy was applied. This analysis rests on the assumption that flutamide does not shorten life expectancy and cancer-specific survival. Radiation therapy was not delivered by current standards of dose and technique.
Interstitial implantation of radioisotopes
Interstitial implantation of radioisotopes (i.e., iodine I 125 [125I], palladium, and iridium Ir 192) done through a transperineal technique with either ultrasound or computed-tomography guidance, is being used in patients with T1 or T2a tumors. Short-term results in these patients are similar to those for radical prostatectomy or EBRT.[32,33]; [34][Level of evidence C3]
Factors for consideration in the use of interstitial implants include the following:
- The implant is performed as outpatient surgery.
- The rate of maintenance of sexual potency with interstitial implants has been reported to be 86% to 92%.[32,34] In contrast, rates of maintenance of sexual potency with radical prostatectomy were 10% to 40% and 40% to 60% with EBRT.
- Typical side effects from interstitial implants that subside with time include urinary tract frequency, urgency, and less commonly, urinary retention.
- Rectal ulceration may also be seen. In one series, a 10% 2-year actuarial genitourinary grade 2 complication rate and a 12% risk of rectal ulceration were seen. This risk decreased with increased operator experience and modification of the implant technique.[32]
Long-term follow-up of these patients is necessary to assess treatment efficacy and side effects.
Retropubic freehand implantation with 125I has been associated with an increased local failure and complication rate [35,36] and is now rarely done.
Photodynamic therapy
Vascular-targeted photodynamic therapy using a photosensitizing agent has been tested in men with low-risk prostate cancer. In the CLIN1001 PCM301 (NCT01310894) randomized trial, 413 men with low-risk cancer (tumor stage T1–T2c, PSA ≤10 ng/mL, generally Gleason score 3 + 3) were randomly assigned in an open-label trial to receive either the photosensitizing agent, padeliporfin (4 mg/kg intravenously [IV] over 10 minutes, and optical fibers inserted into the target area of the prostate, then activated by 753 nm laser light at 150 mW/cm for 22 minutes 15 seconds), or active surveillance.[37] Median time to local disease progression was 28.3 months for patients who received padeliporfin and 14.1 months for patients who were assigned to active surveillance (HR, 0.34; 95% CI, 0.24–0.46; P < .0001).[37][Level of evidence B1] However, the appropriate population for photodynamic therapy may be quite narrow, as it may overtreat men with very low-risk disease and undertreat men with higher-risk disease.[38]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
-
Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 715–26.
-
Consensus conference. The management of clinically localized prostate cancer. JAMA 258 (19): 2727-30, 1987.
-
Epstein JI, Paull G, Eggleston JC, et al.: Prognosis of untreated stage A1 prostatic carcinoma: a study of 94 cases with extended followup. J Urol 136 (4): 837-9, 1986.
-
Thüroff S, Chaussy C, Vallancien G, et al.: High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multicentric study. J Endourol 17 (8): 673-7, 2003.
-
Blana A, Murat FJ, Walter B, et al.: First analysis of the long-term results with transrectal HIFU in patients with localised prostate cancer. Eur Urol 53 (6): 1194-201, 2008.
-
Ficarra V, Novara G: Editorial comment on: first analysis of the long-term results with transrectal HIFU in patients with localized prostate cancer. Eur Urol 53 (6): 1201-2, 2008.
-
Eastham JA: Editorial comment on: first analysis of the long-term results with transrectal HIFU in patients with localized prostate cancer. Eur Urol 53 (6): 1202-3, 2008.
-
Chodak GW, Thisted RA, Gerber GS, et al.: Results of conservative management of clinically localized prostate cancer. N Engl J Med 330 (4): 242-8, 1994.
-
Whitmore WF: Expectant management of clinically localized prostatic cancer. Semin Oncol 21 (5): 560-8, 1994.
-
Shappley WV, Kenfield SA, Kasperzyk JL, et al.: Prospective study of determinants and outcomes of deferred treatment or watchful waiting among men with prostate cancer in a nationwide cohort. J Clin Oncol 27 (30): 4980-5, 2009.
-
Zincke H, Bergstralh EJ, Blute ML, et al.: Radical prostatectomy for clinically localized prostate cancer: long-term results of 1,143 patients from a single institution. J Clin Oncol 12 (11): 2254-63, 1994.
-
Catalona WJ, Bigg SW: Nerve-sparing radical prostatectomy: evaluation of results after 250 patients. J Urol 143 (3): 538-43; discussion 544, 1990.
-
Catalona WJ, Basler JW: Return of erections and urinary continence following nerve sparing radical retropubic prostatectomy. J Urol 150 (3): 905-7, 1993.
-
Paulson DF, Moul JW, Walther PJ: Radical prostatectomy for clinical stage T1-2N0M0 prostatic adenocarcinoma: long-term results. J Urol 144 (5): 1180-4, 1990.
-
Thompson IM, Tangen CM, Paradelo J, et al.: Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 296 (19): 2329-35, 2006.
-
Thompson IM, Tangen CM, Paradelo J, et al.: Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181 (3): 956-62, 2009.
-
Bolla M, van Poppel H, Collette L, et al.: Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet 366 (9485): 572-8, 2005 Aug 13-19.
-
Wilt TJ, Brawer MK, Jones KM, et al.: Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 367 (3): 203-13, 2012.
-
Wilt TJ: The Prostate Cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy with watchful waiting for men with clinically localized prostate cancer. J Natl Cancer Inst Monogr 2012 (45): 184-90, 2012.
-
Wilt TJ, Jones KM, Barry MJ, et al.: Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl J Med 377 (2): 132-142, 2017.
-
Bagshaw MA: External radiation therapy of carcinoma of the prostate. Cancer 45 (7 Suppl): 1912-21, 1980.
-
Forman JD, Zinreich E, Lee DJ, et al.: Improving the therapeutic ratio of external beam irradiation for carcinoma of the prostate. Int J Radiat Oncol Biol Phys 11 (12): 2073-80, 1985.
-
Ploysongsang S, Aron BS, Shehata WM, et al.: Comparison of whole pelvis versus small-field radiation therapy for carcinoma of prostate. Urology 27 (1): 10-6, 1986.
-
Pilepich MV, Bagshaw MA, Asbell SO, et al.: Definitive radiotherapy in resectable (stage A2 and B) carcinoma of the prostate--results of a nationwide overview. Int J Radiat Oncol Biol Phys 13 (5): 659-63, 1987.
-
Amdur RJ, Parsons JT, Fitzgerald LT, et al.: The effect of overall treatment time on local control in patients with adenocarcinoma of the prostate treated with radiation therapy. Int J Radiat Oncol Biol Phys 19 (6): 1377-82, 1990.
-
Seymore CH, el-Mahdi AM, Schellhammer PF: The effect of prior transurethral resection of the prostate on post radiation urethral strictures and bladder neck contractures. Int J Radiat Oncol Biol Phys 12 (9): 1597-600, 1986.
-
Seidenfeld J, Samson DJ, Aronson N, et al.: Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid Rep Technol Assess (Summ) (4): i-x, 1-246, I1-36, passim, 1999.
-
Widmark A, Klepp O, Solberg A, et al.: Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 373 (9660): 301-8, 2009.
-
Asbell SO, Martz KL, Shin KH, et al.: Impact of surgical staging in evaluating the radiotherapeutic outcome in RTOG #77-06, a phase III study for T1BN0M0 (A2) and T2N0M0 (B) prostate carcinoma. Int J Radiat Oncol Biol Phys 40 (4): 769-82, 1998.
-
Roach M, DeSilvio M, Lawton C, et al.: Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 21 (10): 1904-11, 2003.
-
Pollack A: A call for more with a desire for less: pelvic radiotherapy with androgen deprivation in the treatment of prostate cancer. J Clin Oncol 21 (10): 1899-901, 2003.
-
Wallner K, Roy J, Harrison L: Tumor control and morbidity following transperineal iodine 125 implantation for stage T1/T2 prostatic carcinoma. J Clin Oncol 14 (2): 449-53, 1996.
-
D'Amico AV, Coleman CN: Role of interstitial radiotherapy in the management of clinically organ-confined prostate cancer: the jury is still out. J Clin Oncol 14 (1): 304-15, 1996.
-
Ragde H, Blasko JC, Grimm PD, et al.: Interstitial iodine-125 radiation without adjuvant therapy in the treatment of clinically localized prostate carcinoma. Cancer 80 (3): 442-53, 1997.
-
Kuban DA, el-Mahdi AM, Schellhammer PF: I-125 interstitial implantation for prostate cancer. What have we learned 10 years later? Cancer 63 (12): 2415-20, 1989.
-
Fuks Z, Leibel SA, Wallner KE, et al.: The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys 21 (3): 537-47, 1991.
-
Azzouzi AR, Vincendeau S, Barret E, et al.: Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 18 (2): 181-191, 2017.
-
Freedland SJ: Low-risk prostate cancer: to treat or not to treat. Lancet Oncol 18 (2): 156-157, 2017.
Treatment of Stage II Prostate Cancer
Overview
Stage II prostate cancer is defined by the American Joint Committee on Cancer's TNM (tumor, node, metastasis) classification system:[1]
Stage IIA
- cT1a–c, N0, M0, prostate-specific antigen (PSA) ≥10 <20 ng/mL, Gleason ≤6.
- cT2a, N0, M0, PSA ≥10 <20 ng/mL, Gleason ≤6.
- pT2, N0, M0, PSA ≥10 <20 ng/mL, Gleason ≤6.
- cT2b–c, N0, M0, PSA <20 ng/mL, Gleason ≤6.
Stage IIB
- T1–2, N0, M0, PSA <20 ng/mL, Gleason 7.
Stage IIC
- T1–2, N0, M0, PSA <20, Gleason 7 or 8.
Radical prostatectomy, external-beam radiation therapy (EBRT), and interstitial implantation of radioisotopes are each employed in the treatment of stage II prostate cancer with apparently similar therapeutic effects. Radical prostatectomy and radiation therapy yield apparently similar survival rates with as many as 10 years of follow-up. For well-selected patients, radical prostatectomy is associated with a 15-year survival comparable with an age-matched population without prostate cancer.[2] Unfortunately, randomized comparative trials of these treatment methods with prolonged follow-up are lacking.
Patients with a small, palpable cancer (T2a, N0, and M0) fare better than patients in whom the disease involves both sides of the gland (T2c, N0, and M0). Patients proven free of node metastases by pelvic lymphadenectomy fare better than patients in whom this staging procedure is not performed; however, this is the result of selection of patients who have a more favorable prognosis.
Side effects of the various forms of therapy—including impotence, incontinence, and bowel injury—should be considered in determining the type of treatment to employ.
Prostate-specific antigen (PSA) changes as markers of tumor progression
Often, changes in PSA are thought to be markers of tumor progression. Even though a tumor marker or characteristic may be consistently associated with a high risk of prostate cancer progression or death, it may be a very poor predictor of very limited utility in making therapeutic decisions.
Baseline PSA and rate of PSA change were associated with subsequent metastasis or prostate cancer death in a cohort of 267 men with clinically localized prostate cancer who were managed by watchful waiting or active surveillance in the control arm of a randomized trial comparing radical prostatectomy with watchful waiting.[3,4] Nevertheless, the accuracy of classifying men into groups whose cancer remained indolent versus those whose cancer progressed was poor at all examined cut points of PSA or PSA rate of change.
Bisphosphonates and risk of bone metastases
Patients with locally advanced nonmetastatic disease (T2–T4, N0–N1, and M0) are at risk of developing bone metastases. Bisphosphonates are being studied as a strategy to decrease this risk.
Evidence (bisphosphonates and risk of bone metastases):
- A placebo-controlled randomized trial (MRC-PR04) evaluated a 5-year regimen of the first-generation bisphosphonate clodronate in high oral doses (2,080 mg qd). Clodronate therapy had no favorable impact on either time to symptomatic bone metastasis or survival.[5][Level of evidence A1]
Treatment Options for Stage II Prostate Cancer
Treatment options for patients with stage II prostate cancer include the following:
- Watchful waiting or active surveillance/active monitoring.
- Radical prostatectomy.
- External-beam radiation therapy (EBRT) with or without hormonal therapy.
- Interstitial implantation of radioisotopes.
- Ultrasound-guided percutaneous cryosurgery (under clinical evaluation).
- High-intensity focused ultrasound (under clinical evaluation).
- Proton-beam radiation therapy (under clinical evaluation).
- Photodynamic therapy (under clinical evaluation).
Patients with stage II prostate cancer are candidates for clinical trials, including trials of neoadjuvant hormonal therapy followed by radical prostatectomy.
Watchful waiting or active surveillance/active monitoring
Asymptomatic patients of advanced age or with concomitant illness may warrant consideration of careful observation without immediate active treatment.[6,7,8] Watch and wait, observation, expectant management, and active surveillance/active monitoring are terms indicating a strategy that does not employ immediate therapy with curative intent. For more information, see the Treatment Option Overview for Prostate Cancer section.
Evidence (observation with delayed hormonal therapy):
- In a retrospective pooled analysis, 828 men with clinically localized prostate cancer were managed by initial conservative therapy with subsequent hormonal therapy given at the time of symptomatic disease progression.[6]
- Patients with well-differentiated tumors or moderately well-differentiated tumors experienced a disease-specific survival of 87% at 10 years. Overall survival (OS) closely approximated the expected survival among men of similar ages in the general population.
- The decision to treat should be made in the context of the patient's age, associated medical illnesses, and personal desires.
Radical prostatectomy
Radical prostatectomy, usually with pelvic lymphadenectomy (with or without the nerve-sparing technique designed to preserve potency) is the most commonly applied therapy with curative intent.[2,9,10] Radical prostatectomy may be difficult after a transurethral resection of the prostate (TURP).
Because about 40% to 50% of men with clinically organ-confined disease are found to have pathological extension beyond the prostate capsule or surgical margins, the role of postprostatectomy adjuvant radiation therapy has been studied.
Consideration may also be given to postoperative radiation therapy (PORT) for patients who are found to have seminal vesicle invasion by tumor at the time of prostatectomy or who have a detectable level of PSA more than 3 weeks after surgery.[11,12,13] Because the duration of follow-up in available studies is relatively short, the value of PORT has not been determined; however, PORT does reduce local recurrence.[11] Careful treatment planning is necessary to avoid morbidity.
Evidence (radical prostatectomy followed by radiation therapy):
- In a randomized trial of 425 men with pathological T3, N0, M0 disease, postsurgical EBRT (60–64 Gy to the prostatic fossa over 30–32 fractions) was compared with observation.[12][Level of evidence A1]
- The primary end point, metastasis-free survival, could be affected by serial PSA monitoring and resulting metastatic work-up for PSA increase. This could have biased the primary end point in favor of radiation therapy, which was associated with a lower rate of PSA rise. Nevertheless, metastasis-free survival was not statistically different between the two study arms (P = .06). After a median follow-up of about 10.6 years, overall median survival was 14.7 years in the radiation therapy group versus 13.8 years in the observation group (P = .16).
- Although the OS rates were not statistically different, complication rates were substantially higher in the radiation therapy group compared with the observation group: overall complications were 23.8% versus 11.9%, rectal complications were 3.3% versus 0%, and urethral stricture was 17.8% versus 9.5%, respectively.
- After a median follow-up of about 12.5 years, however, OS was better in the radiation therapy arm; hazard ratio (HR)death, 0.72 (95% confidence interval [CI], 0.55–0.96; P = .023). The 10-year estimated survival rates were 74% in the radiation therapy arm and 66% in the control arm. The 10-year estimated metastasis-free survivals were 73% and 65% (P = .016).[13][Level of evidence A1]
Evidence (radical prostatectomy compared directly with watchful waiting/active surveillance/active monitoring and/or external-beam radiation therapy):
- In a randomized clinical trial performed in Sweden in the pre-PSA screening era, 695 men with prostate cancer were randomly assigned to radical prostatectomy versus watchful waiting. Only about 5% of the men in the trial had been diagnosed by PSA screening. Therefore, the men had more extensive local disease than is typically the case in men diagnosed with prostate cancer in the United States.[14,15,16]
- The cumulative overall mortality at 18 years was 56.1% in the radical prostatectomy arm and 68.9% in the watchful waiting study arm (absolute difference, 12.7%; 95% CI, 5.1–20.3 percentage points; relative risk [RR]death, 0.71; 95% CI, 0.59–0.86.[16][Level of evidence A1]
- The cumulative incidence of prostate cancer deaths at 18 years was 17.7% versus 28.7% (absolute difference, 11.0%; 95% CI, 4.5–17.5 percentage points; RRdeath from prostate cancer, 0.56; 95% CI, 0.41–0.77).[16]
- In a post-hoc–subset analysis, the improvement in overall and prostate cancer-specific mortality associated with radical prostatectomy was restricted to men younger than 65 years.
- The Prostate Intervention Versus Observation Trial (PIVOT-1 or VA-CSP-407) is a randomized trial conducted in the PSA screening era that directly compared radical prostatectomy with watchful waiting. From November 1994 through January 2002, 731 men aged 75 years or younger with localized prostate cancer (stage T1–2, NX, M0, with a blood PSA <50 ng/mL) and a life expectancy of at least 10 years were randomly assigned to radical prostatectomy versus watchful waiting.[17,18,19][Level of evidence A1]
- About 50% of the men had palpable tumors.
- After a median follow-up of 12.7 years (range up to about 19.5 years), the all-cause mortality was 61.3% versus 66.8% in the radical-prostatectomy and watchful-waiting study arms, respectively, an absolute difference of 5.5 percentage points (95% CI -1.5 to 12.4) that was not statistically significant (HR, 0.84; 95% CI, 0.70–1.01). Prostate cancer–specific mortality was 7.4% versus 11.4%, and it also was not statistically significant (HR, 0.63; 95% CI, 0.3–1.02).
- Although treatment for disease progression was given more frequently in the observation arm of the study, most such treatment was for asymptomatic, local, or biochemical (PSA) progression.
- As expected, urinary incontinence and erectile/sexual dysfunction was more common in the prostatectomy group for at least 10 years of follow-up. Absolute differences in patient-reported use of absorbent urinary pads was greater in the surgery group by more than 30 percentage points at all time points for at least 10 years. Disease- or treatment-related limitations in activities of daily living were worse with surgery than with observation through 2 years, but then were similar in both study arms.
- In the ProtecT trial (NCT02044172 and ISRCTN20141297), 82,429 men were screened with PSA testing, and 2,664 were diagnosed with clinically localized prostate cancer. Among those diagnosed, 1,643 men (median age 62 years, range 50–69 years) consented to a randomly assigned comparison of active monitoring, radical prostatectomy (nerve-sparing when possible), or external-beam 3D conformal radiation (74 Gy in 37 fractions). The primary end point was prostate cancer-specific mortality.[20]
- With a median follow-up of 10 years, there were a total of 17 deaths from prostate cancer, with no statistically significant differences among the three study arms (P = .48). The 10-year prostate cancer–specific survival rates were 98.8% in the active monitoring arm, 99.0% in the radical prostatectomy arm, and 99.6% radiation therapy arms.[20][Level of evidence A1]
- Likewise, all-cause mortality was nearly identical in all three study arms: 10.9 deaths in the active monitoring arm, 10.1 in the radical prostatectomy arm, and 10.3 in the radiation therapy arm per 1,000 person-years (P = .87).[20][Level of evidence A1]
- There were statistically significant differences in progression to metastatic disease among the treatment arms (33 of 545 men in the active monitoring arm; 13 of 553 men in the radical prostatectomy arm; 16 of 545 men in the radiation therapy arm) that began to emerge after 4 years, but these differences had not translated into any difference in mortality after 10-years of follow-up. Over the course of 10 years, 52% of the patients required active intervention.
- As expected, there were substantial differences in patient-reported outcomes among the three management approaches.[21][Level of evidence A3] A substudy of patient-reported outcomes up to 6 years after randomization included the following:
- Men in the radical prostatectomy study arm had substantial rates of urinary incontinence (e.g., using one or more absorbent pads qd was reported by 46% at 6 months and by 17% at year 6) with very little incontinence in the other two study arms.
- Sexual function was also worse in the radical prostatectomy group (e.g., at 6 months, 12% of men reported erections firm enough for intercourse vs. 22% in the radiation therapy arm and 52% in the active-monitoring arm).
- Bowel function, however, was worse in the radiation therapy arm (e.g., about 5% reported bloody stools at least half the time at 2 years and beyond versus none in the radical prostatectomy and active-monitoring study arms).
External-beam radiation therapy (EBRT) with or without hormonal therapy
EBRT is another treatment option often used with curative intent.[22,23,24,25,26] Definitive radiation therapy should be delayed 4 to 6 weeks after TURP to reduce the incidence of stricture.[27] Adjuvant hormonal therapy should be considered for patients with bulky T2b to T2c tumors.[28]
The role of adjuvant hormonal therapy in patients with locally advanced disease has been analyzed by the Agency for Health Care Policy and Research (now the Agency for Healthcare Research and Quality). Most patients had more advanced disease, but patients with bulky T2b to T2c tumors were included in the studies that were re-evaluating the role of adjuvant hormonal therapy in patients with locally advanced disease.
Evidence (EBRT with or without adjuvant hormonal therapy):
- The Radiation Therapy Oncology Group's (RTOG) trial 7706 (RTOG-7706).[29][Level of evidence A1]
- Prophylactic radiation therapy to clinically or pathologically uninvolved pelvic lymph nodes does not appear to improve OS or prostate cancer-specific survival.
- RTOG-9413 (RTOG-9413 [NCT00769548]) trial.[30,31][Level of evidence B1]
- Although RTOG-9413 showed increased progression-free survival at 4 years for patients who had a 15% estimated risk of lymph node involvement and received whole-pelvic radiation therapy compared with prostate-only radiation therapy, OS and PSA failure rates were not significantly different.
- In a randomized trial, 875 men with locally advanced nonmetastatic prostate cancer (T1b–T2 moderately or poorly differentiated tumors; T3 tumors of any grade) were randomly assigned to receive 3 months of a luteinizing hormone-releasing hormone (LH-RH) agonist plus long-term flutamide (250 mg PO tid) with or without EBRT.[32][Level of evidence A1]
- Nineteen percent of the men had tumor stage T2, and 78% of the men had tumor stage T3. At 10 years, both overall mortality (29.6% vs. 39.4%; 95% CI for the difference, 0.8%–18.8%) and prostate cancer-specific mortality (11.9% vs. 23.9%; 95% CI for the difference, 4.9%–19.1%) favored combined hormonal and radiation therapy.
- Although flutamide might not be considered a standard hormonal monotherapy in the setting of T2 or T3 tumors, radiation therapy provided a disease-free survival or tumor-specific survival advantage even though this monotherapy was applied. This analysis rests on the assumption that flutamide does not shorten life expectancy and cancer-specific survival. Radiation therapy was not delivered by current standards of dose and technique.
- Another trial compared androgen deprivation therapy (ADT: an LH-RH agonist or orchiectomy) with ADT plus radiation therapy (65–69 Gy to the prostate by 4-field box technique, including 45 Gy to the whole pelvis, seminal vesicles, and external/internal iliac nodes unless the lymph nodes were histologically negative). This trial, NCIC CTG PR.3/MRC UKPRO7 [NCT00002633], from the National Cancer Institute of Canada randomly assigned 1,205 patients with high-risk (PSA >40 ng/mL or PSA >20 ng/mL and Gleason score ≥8), T2 (12%–13% of the patients), T3 (83% of the patients), and T4 (4%–5% of the patients) with clinical or pathologically staged N0, M0 disease.[33,34][Level of evidence A1]
- At a median follow-up of 8 years (maximum, 13 years), OS was superior in the ADT-plus-radiation therapy group (HRdeath, 0.77; 95% CI, 0.57–0.85, P = .001). The OS rate at 10 years was 55% for the ADT-plus-radiation therapy group versus 49% for the ADT-alone group.
- Although radiation therapy had the expected bowel and urinary side effects, quality of life was the same in each study group by 24 months and beyond.[35]
- A meta-analysis of randomized clinical trial evidence comparing radiation therapy with radiation therapy plus prolonged androgen suppression has been published. The meta-analysis found a difference in 5-year OS in favor of radiation therapy plus continued androgen suppression (LH-RH agonist or orchiectomy) as compared with radiation therapy alone (HR, 0.631; 95% CI, 0.479–0.831).[28][Level of evidence A1]
- In a randomized, prospective clinical trial, 18 months of androgen suppression with an LH-RH agonist appears to have provided results that were similar to 36 months with respect to OS and disease-specific survival.[36][Level of evidence A1] In a multicenter trial, 630 men with stage II to stage IVA cancer (clinical stage T3–T4, or PSA >20 ng/ml, or Gleason score >7) received 70 Gy of radiation in 35 fractions plus a total of either 18 or 36 months of goserelin acetate.
- With a median follow-up of 9.4 years, OS was nearly identical in each study arm (62% at 10 years; HRdeath, 1.02; 95% CI, 0.81–1.29; P = .8), as was prostate cancer–specific survival (HRprostate death, 0.95; 95% CI, 0.58–0.55; P = .8).
- Global quality of life was nearly identical on both study arms, but sexual activity and interest in sex was moderately better in the 18-month arm.[36][Level of evidence A3]
- A meta-analysis of seven randomized controlled trials comparing early hormonal treatment (adjuvant or neoadjuvant) to deferred hormonal treatment (LH-RH agonists and/or antiandrogens) in patients with locally advanced prostate cancer, whether treated with prostatectomy, radiation therapy, or watchful waiting or active surveillance/active monitoring, showed improved overall mortality for patients receiving early treatment (RR, 0.86; 95% CI, 0.82–0.91).[37][Level of evidence A1]
- Short-term neoadjuvant−androgen therapy administered before and during radiation therapy has shown benefit in at least some patients with clinically localized prostate cancer. In an open-label, randomized trial (RTOG-9408 [NCT00002597]), 1,979 men with nonmetastatic stage T1b–c, T2a, or T2b tumors and a PSA level of 20 ng/mL or less were randomly assigned to receive radiation therapy (66.6 Gy prostate dose in 1.8 Gy daily fractions) with or without 4 months of ADT (flutamide 250 mg PO tid plus either monthly goserelin 3.6 mg subcutaneously (SQ) or leuprolide 7.5 mg intramuscularly), beginning 2 months before radiation therapy. Median follow-up was about 9 years.[38][Level of evidence A1]
- The 10-year OS rate was 57% in the radiation-only group versus 62% in the combined-therapy group (HRdeath, 1.17; 95% CI, 1.01–1.35; P = .03).
- In a post-hoc analysis, there was no statistically significant interaction between the treatment effect and baseline-risk category of the patients. However, there appeared to be little, if any, benefit associated with combined therapy in the lowest-risk category of patients (Gleason score ≤6; PSA ≤10 ng/mL; and clinical stage ≤T2a).
- The OS benefit was most apparent in men with intermediate-risk tumors (Gleason score 7; or Gleason score ≤6 and PSA >10 ng/mL; or clinical stage T2b).
- The duration of neoadjuvant hormonal therapy has been tested in a randomized trial (TROG 96.01 [ACTRN12607000237482]) involving 818 men with locally advanced (T2b, T2c, T3, and T4) nonmetastatic cancer treated with radiation therapy (i.e., 66 Gy in 2 Gy daily fractions to the prostate and seminal vesicles but not including regional lymph nodes).[39] In an open-label design, patients were randomly assigned to receive radiation therapy alone, 3 months of neoadjuvant androgen deprivation therapy (NADT) (goserelin 3.6 mg SQ each month plus flutamide 250 mg PO tid) for 2 months before and during radiation, or 6 months of NADT for 5 months before and during radiation.[39][Level of evidence A1]
- After a median follow-up of 10.6 years, there were no statistically significant differences between the radiation-alone group and the radiation-plus-3-months-of NADT group.
- However, the 6-month NADT arm showed better prostate–cancer-specific mortality and overall mortality than the radiation-alone group; 10-year all-cause mortality 29.2% versus 42.5% (HR, 0.63; 95% CI, 0.48–0.83, P = .0008).
- The duration of neoadjuvant hormonal therapy was tested in another trial (RTOG-9910 [NCT00005044]) of 1,489 eligible men with intermediate-risk prostate cancer (T1b–4, Gleason score 2–6, and PSA >10 but ≤100 ng/mL; T1b–4, Gleason score 7, and PSA <20; or T1b–1c, Gleason score 8–10, and PSA <20) and no evidence of metastases. The men were randomly assigned to receive short-course neoadjuvant–androgen suppression (an LH-RH agonist plus bicalutamide or flutamide for 8 weeks before and 8 weeks during radiation therapy) or long-course neoadjuvant–androgen suppression (28 weeks before and 8 weeks during radiation therapy). Both groups received 70.2 Gy radiation in 39 daily fractions to the prostate and 46.8 Gy to the iliac lymph nodes.[40][Level of evidence A1]
- After a median of 9.4 years, 10-year prostate-specific mortality, the primary end point, was low in both study arms: 5% versus 4% (HR, 0.81; 95% CI, 0.48–1.39).[40][Level of evidence A1]
- No statistically significant differences in overall mortality or in locoregional disease progression were found.[40][Level of evidence A1]
- There was also no apparent differential effect of androgen suppression duration among any of the risk-group subsets.
- Addition of androgen suppression therapy to EBRT may benefit men who are at an elevated risk of disease recurrence and death from prostate cancer (RTOG-9202 [NCT00767286]).
Interstitial implantation of radioisotopes
Interstitial implantation of radioisotopes (i.e., iodine I 125 [125I], palladium, and iridium), using a transperineal technique with either ultrasound or computed tomography guidance, is being done in patients with T1 or T2a tumors. Short-term results in these patients are similar to those for radical prostatectomy or EBRT.[41,42]; [43][Level of evidence C3]
Factors for consideration in the use of interstitial implants include the following:
- The implant is performed as outpatient surgery.
- The rate of maintenance of sexual potency with interstitial implants has been reported to be 86% to 92%.[41,43] In contrast, rates of maintenance of sexual potency with radical prostatectomy were 10% to 40% and 40% to 60% with EBRT.
- Typical side effects from interstitial implants that are seen in most patients but subside with time include urinary tract frequency, urgency, and less commonly, urinary retention.
- Rectal ulceration may also be seen.[41] In one series, a 10% 2-year actuarial genitourinary grade 2 complication rate and a 12% risk of rectal ulceration were seen. This risk decreased with increased operator experience and modification of the implant technique.[44]
Long-term follow-up of these patients is necessary to assess treatment efficacy and side effects.
Retropubic freehand implantation with 125I has been associated with an increased local failure and complication rate [44,45] and is now rarely done.
Ultrasound-guided percutaneous cryosurgery
Cryosurgery is a surgical technique that involves destruction of prostate cancer cells by intermittent freezing of the prostate with cryoprobes followed by thawing.[46][Level of evidence C1]; [47,48][Level of evidence C3] Cryosurgery is less well established than standard prostatectomy, and long-term outcomes are not as well established as with prostatectomy or radiation therapy. Serious toxic effects include:
- Bladder outlet injury.
- Urinary incontinence.
- Sexual impotence.
- Rectal injury.
The frequency of other side effects and the probability of cancer control at 5 years' follow-up have varied among reporting centers, and series are small compared with surgery and radiation therapy.[47,48]
High-intensity focused ultrasound
High-intensity focused ultrasound has been reported in case series to produce good local disease control. However, it has not been directly compared with more standard therapies, and experience with it is more limited.[49,50,51]
Proton-beam radiation therapy
There is growing interest in the use of proton-beam radiation therapy for the treatment of prostate cancer. Although the dose distribution of this form of charged-particle radiation has the potential to improve the therapeutic ratio of prostate radiation, allowing for an increase in dose to the tumor without a substantial increase in side effects, no randomized controlled trials have been reported that compare its efficacy and toxicity with those of other forms of radiation therapy.
Photodynamic therapy
Vascular-targeted photodynamic therapy using a photosensitizing agent has been tested in men with low-risk prostate cancer. In the CLIN1001 PCM301 (NCT01310894) randomized trial, 413 men with low-risk cancer (tumor stage T1–T2c, PSA ≤10 ng/mL, generally Gleason score 3 + 3) were randomly assigned in an open-label trial to receive either the photosensitizing agent, padeliporfin (4 mg/kg IV over 10 minutes, and optical fibers inserted into the target area of the prostate, then activated by 753 nm laser light at 150 mW/cm for 22 minutes 15 seconds), or active surveillance.[52] Median time to local disease progression was 28.3 months for patients who received padeliporfin and 14.1 months for patients who were assigned to active surveillance (HR, 0.34; 95% CI, 0.24–0.46; P < .0001).[52][Level of evidence B1] However, the appropriate population for photodynamic therapy may be quite narrow, as it may overtreat men with very low-risk disease and undertreat men with higher-risk disease.[53]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
-
Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 715–26.
-
Zincke H, Bergstralh EJ, Blute ML, et al.: Radical prostatectomy for clinically localized prostate cancer: long-term results of 1,143 patients from a single institution. J Clin Oncol 12 (11): 2254-63, 1994.
-
Fall K, Garmo H, Andrén O, et al.: Prostate-specific antigen levels as a predictor of lethal prostate cancer. J Natl Cancer Inst 99 (7): 526-32, 2007.
-
Parekh DJ, Ankerst DP, Thompson IM: Prostate-specific antigen levels, prostate-specific antigen kinetics, and prostate cancer prognosis: a tocsin calling for prospective studies. J Natl Cancer Inst 99 (7): 496-7, 2007.
-
Mason MD, Sydes MR, Glaholm J, et al.: Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873). J Natl Cancer Inst 99 (10): 765-76, 2007.
-
Chodak GW, Thisted RA, Gerber GS, et al.: Results of conservative management of clinically localized prostate cancer. N Engl J Med 330 (4): 242-8, 1994.
-
Whitmore WF: Expectant management of clinically localized prostatic cancer. Semin Oncol 21 (5): 560-8, 1994.
-
Shappley WV, Kenfield SA, Kasperzyk JL, et al.: Prospective study of determinants and outcomes of deferred treatment or watchful waiting among men with prostate cancer in a nationwide cohort. J Clin Oncol 27 (30): 4980-5, 2009.
-
Catalona WJ, Bigg SW: Nerve-sparing radical prostatectomy: evaluation of results after 250 patients. J Urol 143 (3): 538-43; discussion 544, 1990.
-
Catalona WJ, Basler JW: Return of erections and urinary continence following nerve sparing radical retropubic prostatectomy. J Urol 150 (3): 905-7, 1993.
-
Paulson DF, Moul JW, Walther PJ: Radical prostatectomy for clinical stage T1-2N0M0 prostatic adenocarcinoma: long-term results. J Urol 144 (5): 1180-4, 1990.
-
Thompson IM, Tangen CM, Paradelo J, et al.: Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 296 (19): 2329-35, 2006.
-
Thompson IM, Tangen CM, Paradelo J, et al.: Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181 (3): 956-62, 2009.
-
Holmberg L, Bill-Axelson A, Helgesen F, et al.: A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med 347 (11): 781-9, 2002.
-
Bill-Axelson A, Holmberg L, Ruutu M, et al.: Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 352 (19): 1977-84, 2005.
-
Bill-Axelson A, Holmberg L, Garmo H, et al.: Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 370 (10): 932-42, 2014.
-
Wilt TJ, Brawer MK, Jones KM, et al.: Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 367 (3): 203-13, 2012.
-
Wilt TJ: The Prostate Cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy with watchful waiting for men with clinically localized prostate cancer. J Natl Cancer Inst Monogr 2012 (45): 184-90, 2012.
-
Wilt TJ, Jones KM, Barry MJ, et al.: Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl J Med 377 (2): 132-142, 2017.
-
Hamdy FC, Donovan JL, Lane JA, et al.: 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 375 (15): 1415-1424, 2016.
-
Donovan JL, Hamdy FC, Lane JA, et al.: Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 375 (15): 1425-1437, 2016.
-
Bagshaw MA: External radiation therapy of carcinoma of the prostate. Cancer 45 (7 Suppl): 1912-21, 1980.
-
Forman JD, Zinreich E, Lee DJ, et al.: Improving the therapeutic ratio of external beam irradiation for carcinoma of the prostate. Int J Radiat Oncol Biol Phys 11 (12): 2073-80, 1985.
-
Ploysongsang S, Aron BS, Shehata WM, et al.: Comparison of whole pelvis versus small-field radiation therapy for carcinoma of prostate. Urology 27 (1): 10-6, 1986.
-
Pilepich MV, Bagshaw MA, Asbell SO, et al.: Definitive radiotherapy in resectable (stage A2 and B) carcinoma of the prostate--results of a nationwide overview. Int J Radiat Oncol Biol Phys 13 (5): 659-63, 1987.
-
Amdur RJ, Parsons JT, Fitzgerald LT, et al.: The effect of overall treatment time on local control in patients with adenocarcinoma of the prostate treated with radiation therapy. Int J Radiat Oncol Biol Phys 19 (6): 1377-82, 1990.
-
Seymore CH, el-Mahdi AM, Schellhammer PF: The effect of prior transurethral resection of the prostate on post radiation urethral strictures and bladder neck contractures. Int J Radiat Oncol Biol Phys 12 (9): 1597-600, 1986.
-
Seidenfeld J, Samson DJ, Aronson N, et al.: Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid Rep Technol Assess (Summ) (4): i-x, 1-246, I1-36, passim, 1999.
-
Asbell SO, Martz KL, Shin KH, et al.: Impact of surgical staging in evaluating the radiotherapeutic outcome in RTOG #77-06, a phase III study for T1BN0M0 (A2) and T2N0M0 (B) prostate carcinoma. Int J Radiat Oncol Biol Phys 40 (4): 769-82, 1998.
-
Roach M, DeSilvio M, Lawton C, et al.: Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 21 (10): 1904-11, 2003.
-
Pollack A: A call for more with a desire for less: pelvic radiotherapy with androgen deprivation in the treatment of prostate cancer. J Clin Oncol 21 (10): 1899-901, 2003.
-
Widmark A, Klepp O, Solberg A, et al.: Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 373 (9660): 301-8, 2009.
-
Warde P, Mason M, Ding K, et al.: Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 378 (9809): 2104-11, 2011.
-
Mason MD, Parulekar WR, Sydes MR, et al.: Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. J Clin Oncol 33 (19): 2143-50, 2015.
-
Brundage M, Sydes MR, Parulekar WR, et al.: Impact of Radiotherapy When Added to Androgen-Deprivation Therapy for Locally Advanced Prostate Cancer: Long-Term Quality-of-Life Outcomes From the NCIC CTG PR3/MRC PR07 Randomized Trial. J Clin Oncol 33 (19): 2151-7, 2015.
-
Nabid A, Carrier N, Martin AG, et al.: Duration of Androgen Deprivation Therapy in High-risk Prostate Cancer: A Randomized Phase III Trial. Eur Urol 74 (4): 432-441, 2018.
-
Boustead G, Edwards SJ: Systematic review of early vs deferred hormonal treatment of locally advanced prostate cancer: a meta-analysis of randomized controlled trials. BJU Int 99 (6): 1383-9, 2007.
-
Jones CU, Hunt D, McGowan DG, et al.: Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365 (2): 107-18, 2011.
-
Denham JW, Steigler A, Lamb DS, et al.: Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12 (5): 451-9, 2011.
-
Pisansky TM, Hunt D, Gomella LG, et al.: Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol 33 (4): 332-9, 2015.
-
Wallner K, Roy J, Harrison L: Tumor control and morbidity following transperineal iodine 125 implantation for stage T1/T2 prostatic carcinoma. J Clin Oncol 14 (2): 449-53, 1996.
-
D'Amico AV, Coleman CN: Role of interstitial radiotherapy in the management of clinically organ-confined prostate cancer: the jury is still out. J Clin Oncol 14 (1): 304-15, 1996.
-
Ragde H, Blasko JC, Grimm PD, et al.: Interstitial iodine-125 radiation without adjuvant therapy in the treatment of clinically localized prostate carcinoma. Cancer 80 (3): 442-53, 1997.
-
Kuban DA, el-Mahdi AM, Schellhammer PF: I-125 interstitial implantation for prostate cancer. What have we learned 10 years later? Cancer 63 (12): 2415-20, 1989.
-
Fuks Z, Leibel SA, Wallner KE, et al.: The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys 21 (3): 537-47, 1991.
-
Robinson JW, Saliken JC, Donnelly BJ, et al.: Quality-of-life outcomes for men treated with cryosurgery for localized prostate carcinoma. Cancer 86 (9): 1793-801, 1999.
-
Donnelly BJ, Saliken JC, Ernst DS, et al.: Prospective trial of cryosurgical ablation of the prostate: five-year results. Urology 60 (4): 645-9, 2002.
-
Aus G, Pileblad E, Hugosson J: Cryosurgical ablation of the prostate: 5-year follow-up of a prospective study. Eur Urol 42 (2): 133-8, 2002.
-
Blana A, Murat FJ, Walter B, et al.: First analysis of the long-term results with transrectal HIFU in patients with localised prostate cancer. Eur Urol 53 (6): 1194-201, 2008.
-
Ficarra V, Novara G: Editorial comment on: first analysis of the long-term results with transrectal HIFU in patients with localized prostate cancer. Eur Urol 53 (6): 1201-2, 2008.
-
Eastham JA: Editorial comment on: first analysis of the long-term results with transrectal HIFU in patients with localized prostate cancer. Eur Urol 53 (6): 1202-3, 2008.
-
Azzouzi AR, Vincendeau S, Barret E, et al.: Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 18 (2): 181-191, 2017.
-
Freedland SJ: Low-risk prostate cancer: to treat or not to treat. Lancet Oncol 18 (2): 156-157, 2017.
Treatment of Stage III Prostate Cancer
Overview
Stage III prostate cancer is defined by the American Joint Committee on Cancer's TNM (tumor, node, metastasis) classification system:[1]
Stage IIIA
- T1–2, N0, M0, prostate-specific antigen (PSA) ≥20, Gleason ≤6–8.
Stage IIIB
- T3–4, N0, M0, any PSA, Gleason ≤6–8.
Stage IIIC
- Any T, N0, M0, any PSA, Gleason 9 or 10.
Extraprostatic extension with microscopic bladder neck invasion (T4) is included with T3a.
External-beam radiation therapy (EBRT), interstitial implantation of radioisotopes, and radical prostatectomy are used to treat stage III prostate cancer.[2] Prognosis is greatly affected by whether regional lymph nodes are evaluated and proven not to be involved.
EBRT using a linear accelerator is the most common treatment for patients with stage III prostate cancer, and large series support its success in achieving local disease control and disease-free survival (DFS).[3,4] The results of radical prostatectomy in stage III patients are greatly inferior compared with results in patients with stage II cancer. Interstitial implantation of radioisotopes is technically difficult in large tumors.
The patient's symptoms related to cancer, age, and coexisting medical illnesses should be considered before deciding on a therapeutic plan. In a series of 372 patients treated with radiation therapy and followed for 20 years, 47% eventually died of prostate cancer, but 44% died of intercurrent illnesses without evidence of prostate cancer.[4]
Treatment Options for Stage III Prostate Cancer
Treatment options for patients with stage III prostate cancer include the following:
- External-beam radiation therapy (EBRT) with or without hormonal therapy.
- Hormonal manipulations (with or without radiation therapy).
- Radical prostatectomy with or without EBRT.
- Watchful waiting or active surveillance/active monitoring.
External-beam radiation therapy (EBRT) with or without hormonal therapy
EBRT alone,[3,4,5,6,7] luteinizing hormone-releasing hormone (LH-RH) agonist, or orchiectomy, in addition to EBRT, should be considered.[8,9,10,11,12,13,14,15,16] Definitive radiation therapy should be delayed until 4 to 6 weeks after transurethral resection to reduce the incidence of stricture.[17]
Hormonal therapy should be considered in conjunction with radiation therapy especially in men who do not have underlying moderate or severe comorbidities.[8,9] Several studies have investigated its use in patients with locally advanced disease.
Evidence (EBRT with or without hormonal therapy):
- Although patients in the Radiation Therapy Oncology Group (RTOG) RTOG-9413 trial (NCT00769548) showed a 15% estimated risk of lymph node involvement and received whole-pelvic radiation therapy compared with prostate-only radiation therapy, overall survival (OS) and PSA failure rates were not significantly different.[18]; [19][Level of evidence B1]
- In a randomized trial, 875 men with locally advanced nonmetastatic prostate cancer (T1b–T2 moderately or poorly differentiated tumors; T3 tumors of any grade) were randomly assigned to receive 3 months of an LH-RH agonist plus long-term flutamide (250 mg PO tid) with or without EBRT. Nineteen percent of the men had tumor stage T2, and 78% of the men had stage T3.[20][Level of evidence A1]
- At 10 years, both overall mortality (29.6% vs. 39.4%; 95% confidence interval [CI] for the difference, 0.8%–8.8%) and the prostate cancer-specific mortality (11.9% vs. 23.9%; 95% CI for the difference, 4.9%–19.1%) favored combined hormonal and radiation therapy.
- Although flutamide might not be considered a standard hormonal monotherapy in the setting of T2 or T3 tumors, radiation therapy provided a DFS or tumor-specific survival advantage even though this monotherapy was applied. This analysis rests on the assumption that flutamide does not shorten life expectancy and cancer-specific survival. Radiation therapy was not delivered by current standards of dose and technique.
- Another trial compared androgen deprivation therapy (ADT: an LH-RH agonist or orchiectomy) to ADT plus radiation therapy (65–69 Gy to the prostate by 4-field box technique, including 45 Gy to the whole pelvis, seminal vesicles, and external/internal iliac nodes unless the lymph nodes were histologically negative). This trial (NCIC CTG PR.3/MRC UKPRO7 [NCT00002633]) from the National Cancer Institute of Canada, randomly assigned 1,205 patients with high-risk (PSA >40 ng/mL or PSA >20 ng/mL and Gleason score ≥8), T2 (12%–13% of the patients), T3 (83% of the patients), and T4 (4%–5% of the patients) with clinical or pathologically staged N0, M0 disease.[21,22][Level of evidence A1]
- At a median follow-up of 8 years (maximum, 13 years), OS was superior in the ADT-plus-radiation therapy group (hazard ratio [HR]death, 0.77; 95% CI, 0.57–0.85, P = .001). The OS rate at 10 years was 55% for the ADT-plus-radiation therapy group versus 49% for the ADT-alone group.
- Although radiation therapy had the expected bowel and urinary side effects, quality of life (QOL) was the same in each study group by 24 months and beyond.[23]
- The RTOG performed a prospective randomized trial (RTOG-8531) in patients with T3, N0, or any T, N1, M0 disease who received prostatic and pelvic radiation therapy and then were randomly assigned to receive immediate adjuvant goserelin or observation with administration of goserelin at time of relapse. In patients assigned to receive adjuvant goserelin, the drug was started during the last week of the radiation therapy course and was continued indefinitely or until signs of progression.[24][Level of evidence A1]
- The actuarial 10-year OS rate for the entire population of 945 analyzable patients was 49% on the adjuvant arm versus 39% on the observation arm (P = .002). There was also an improved actuarial 10-year local failure rate (23% vs. 38%, P < .001).
- A similar trial was performed by the European Organisation for Research and Treatment of Cancer (EORTC). Patients with T1, T2 (World Health Organization grade 3), N0–NX or T3, T4, N0 disease were randomly assigned to receive either pelvic/prostate radiation therapy or identical radiation therapy and adjuvant goserelin (with cyproterone acetate for 1 month) starting with radiation therapy and continuing for 3 years. The 401 patients available for analysis were followed for a median of 9.1 years.[10,25][Levels of evidence A1 and B1]
- The Kaplan-Meier estimates of OS rates at 10 years were 58.1% in the adjuvant goserelin arm and 39.8% in the radiation alone arm (P = .0004). Similarly, 10-year DFS rates (47.7% vs. 22.7%, P < .0001) and local control rates (94.0% vs. 76.5%, P < .001) favored the adjuvant arm.[10,25]
- Two smaller studies, with 78 and 91 patients each, have shown similar results.[26,27]
- The role of adjuvant hormonal therapy in patients with locally advanced disease has been analyzed by the Agency for Health Care Policy and Research (AHCPR; now the Agency for Healthcare Research and Quality). Randomized clinical trial evidence comparing radiation therapy with radiation therapy with prolonged androgen suppression (with an LH-RH agonist or orchiectomy) was evaluated in a meta-analysis. Most patients had more advanced disease, but patients with bulky T2b tumors were included in the study.[11][Level of evidence A1]
- The meta-analysis found a difference in 5-year OS in favor of radiation therapy plus continued androgen suppression compared with radiation therapy alone (HR, 0.631; 95% CI, 0.479–0.831).[11]
- Additionally, the RTOG did a study (RTOG-8610) in patients with bulky local disease (T2b, T2c, T3, or T4), with or without nodal involvement below the common iliac chain: 456 men were randomly assigned to receive either radiation therapy alone or radiation therapy with androgen ablation, which was started 8 weeks before radiation therapy and continued for 16 weeks. This trial assessed only short-term hormonal therapy, not long-term therapy, as the studies analyzed by the AHCPR did.[12,28]
- At 10 years, OS was not statistically significantly different; however, disease-specific mortality rates (23% vs. 36%) and DFS rates (11% vs. 3%) favored the combined treatment arm.[12][Level of evidence A1]
- A subset analysis of the RTOG-8610 trial and the RTOG-8531 trial that involved 575 patients with T3, N0, M0 disease indicated that long-term hormones compared with short-term hormones resulted in improved biochemical DFS and cause-specific survival.[29]
- This finding was confirmed by RTOG-9202 (NCT00767286), which reported that radiation therapy plus 28 months of androgen deprivation resulted in longer 10-year disease-specific survival rates (23% vs. 13%; P < .0001) but not OS rates (53.9% vs. 51.6%; P = .36).[13]
- An unplanned post-hoc subgroup analysis found increased OS with longer androgen deprivation (28 months vs. 4 months) (45% vs. 32%; P = .0061) in men with high-grade cancers and Gleason scores of 8 through 10.
- In a randomized, prospective clinical trial, 18 months of androgen suppression with an LH-RH agonist appears to have provided results that were similar to 36 months with respect to OS and disease-specific survival.[30][Level of evidence A1] In the trial, 630 men with stage II to stage IVA cancer (clinical stage T3–T4, or PSA >20 ng/ml, or Gleason score >7) received 70 Gy of radiation in 35 fractions alone plus a total of either 18 or 36 months of goserelin acetate.
- With a median follow-up of 9.4 years, OS was nearly identical in each study arm (62% at 10 years; HRdeath, 1.02; 95% CI, 0.81–1.29, P = .8), as was prostate cancer–specific survival (HRprostate death, 0.95; 95% CI, 0.58–1.55, P = .8).
- Global quality of life was nearly identical on both study arms, but sexual activity and interest in sex was moderately better in the 18-month arm.[30][Level of evidence A3]
- Likewise, a meta-analysis of seven randomized controlled trials comparing early hormonal treatment (adjuvant or neoadjuvant) with deferred hormonal treatment (LH-RH agonists and/or antiandrogens) in patients with locally advanced prostate cancer, whether treated by prostatectomy, radiation therapy, or watchful waiting or active surveillance/active monitoring, showed improved overall mortality for patients receiving early treatment (relative risk, 0.86; 95% CI, 0.82–0.91).[31][Level of evidence A1]
- The duration of neoadjuvant hormonal therapy has been tested in a randomized trial (TROG 96.01 [ACTRN12607000237482]) involving 818 men with locally advanced (T2b, T2c, T3, and T4) nonmetastatic cancer treated with radiation therapy (i.e., 66 Gy in 2 Gy daily fractions to the prostate and seminal vesicles but not including regional lymph nodes). In an open-label design, patients were randomly assigned to radiation therapy alone, 3 months of neoadjuvant androgen deprivation therapy (NADT) (goserelin 3.6 mg subcutaneously each month plus flutamide 250 mg PO tid) for 2 months before and during radiation, or 6 months of NADT for 5 months before and during radiation.[14][Level of evidence A1]
- After a median follow-up of 10.6 years, there were no statistically significant differences between the radiation-alone group and the radiation plus 3 months of NADT group.
- However, the 6-month NADT arm showed better prostate cancer-specific mortality and overall mortality than radiation alone; 10-year all-cause mortality 29.2% versus 42.5% (HR, 0.63; 95% CI, 0.48–0.83, P = .0008).
- The duration of neoadjuvant hormonal therapy was tested in another trial (RTOG-9910 [NCT00005044]) of 1,489 eligible men with intermediate-risk prostate cancer (T1b–4, Gleason score 2–6, and PSA >10 but ≤100 ng/mL; T1b–4, Gleason score 7, and PSA <20; or T1b–1c, Gleason score 8–10, and PSA <20) and no evidence of metastases. The men were randomly assigned to receive short-course neoadjuvant–androgen suppression (an LH-RH agonist plus bicalutamide or flutamide for 8 weeks before and 8 weeks during radiation therapy) or long-course neoadjuvant–androgen suppression (28 weeks before and 8 weeks during radiation therapy). Both groups received 70.2 Gy radiation in 39 daily fractions to the prostate and 46.8 Gy to the iliac lymph nodes.[32][Level of evidence A1]
- After a median of 9.4 years, 10-year prostate-specific mortality, the primary end point, was low in both study arms: 5% versus 4% (HR, 0.81; 95% CI, 0.48–1.39).[32][Level of evidence A1]
- No statistically significant differences in overall mortality or in locoregional disease progression were found.[32][Level of evidence A1]
- There was also no apparent differential effect of androgen suppression duration among any of the risk-group subsets.
Hormonal manipulations (with or without radiation therapy)
Hormonal manipulations (orchiectomy or LH-RH agonists) may be used in the treatment of stage III prostate cancer.[33][Level of evidence A1]
Some data suggest that the efficacy of orchiectomy or LH-RH agonists may be enhanced by the addition of abiraterone acetate in men with locally advanced tumors. In the randomized, open-label, STAMPEDE trial (NCT00268476) trial, 1,917 men (about 95% newly diagnosed; about 50% had metastatic disease and about 50% had locally advanced or node-positive disease) were treated with ADT alone or ADT plus abiraterone acetate (1,000 mg PO qd) and prednisolone (5 mg PO qd).[34] Local radiation therapy was mandated after 6 to 9 months for men with node-negative nonmetastatic disease and optional for those with node-positive nonmetastatic disease. Hormone therapy was curtailed at 2 years or until progression. Radiation therapy was planned in about 40% of the study participants.
- With a median follow-up of 40 months, the 3-year OS rate was 83% in the abiraterone study group compared with 76% in the ADT-only study group (HRdeath, 0.63; 95% CI, 0.52–0.76; P < .001).[34][Level of evidence A1] Although there was no clear evidence of heterogeneity in relative treatment differences in metastatic disease versus nonmetastatic disease, absolute differences were much smaller in men with nonmetastatic disease and not statistically significant, perhaps because of the short follow-up (HRdeath, 0.75; 95% CI, 0.49–1.18).
- The main additional differences in toxicity associated with abiraterone compared with ADT alone were hypertension (5% vs. 1%), mild increase in blood aminotransferase levels (6% vs. <1%), and respiratory disorders (5% vs. 2%).
Antiandrogen monotherapy has also been evaluated in men with locally advanced prostate cancer as an alternative to castration.
Evidence (nonsteroidal antiandrogen monotherapy vs. surgical or medical castration):
- A systematic evidence review compared nonsteroidal antiandrogen monotherapy with surgical or medical castration from 11 randomized trials in 3,060 men with locally advanced, metastatic, or recurrent disease after local therapy.[35] Use of nonsteroidal antiandrogens as monotherapy decreased OS and increased the rate of clinical progression and treatment failure.[35][Level of evidence A1]
Evidence (orchiectomy vs. LH-RH agonist):
- In a randomized equivalence study involving 480 men with locally advanced (T3 and T4) disease, those who were treated with castration had a median OS of 70 months, whereas those treated with bicalutamide (150 mg qd) had a median OS of 63.5 months (HR, 1.05; 95% CI, 0.81–1.36); these results failed to meet the prespecified criteria for equivalence.[36][Level of evidence A1]
Immediate versus deferred hormonal therapy
In patients who are not candidates for or who are unwilling to undergo radical prostatectomy or radiation therapy, immediate hormonal therapy has been compared with deferred treatment (i.e., watchful waiting or active surveillance/active monitoring with hormonal therapy at progression).
Evidence (immediate vs. deferred hormonal therapy):
- A randomized trial looked at immediate hormonal treatment (orchiectomy or LH-RH agonist) versus deferred treatment in men with locally advanced or asymptomatic metastatic prostate cancer.[33][Level of evidence A1]
- Initial results showed better OS and prostate cancer-specific survival with the immediate treatment. This subsequently lost statistical significance as was recorded in abstract form.[37]
- The incidence of pathological fractures, spinal cord compression, and ureteric obstruction were also lower in the immediate treatment arm.
- In another trial, 197 men with stage III or stage IV prostate cancer were randomly assigned to receive bilateral orchiectomy at diagnosis or at the time of symptomatic progression (or at the time of new metastases that were deemed likely to cause symptoms).[38][Level of evidence A1]
- No statistically significant difference in OS was seen over a 12-year period of follow-up.
- In the EORTC-30891 trial (NCT01819285), 985 patients newly diagnosed with prostate cancer, stage T0–4, N0–2, M0, and a median age of 73 years were randomly assigned to receive androgen deprivation, either immediately or on symptomatic disease progression. The study was designed to demonstrate the noninferiority of deferred treatment as compared with immediate treatment in relation to OS.[39][Level of evidence A1]
- At a median follow-up of 7.8 years, approximately 50% of the patients in the deferred treatment group had initiated androgen deprivation.
- The median OS in the immediate treatment group was 7.4 years, and, in the deferred treatment group, it was 6.5 years, corresponding to a mortality HR of 1.25 (95% CI, 1.05–1.48), which failed to meet the criteria for noninferiority.
Continuous versus intermittent hormonal therapy
When used as the primary therapy for patients with stage III or stage IV prostate cancer, androgen suppression with hormonal therapy is usually given continuously until there is disease progression. Some investigators have proposed intermittent androgen suppression as a strategy to attain maximal tumor cytoreduction followed by a period without therapy to allow tumor repopulation by hormone-sensitive cells. Theoretically, this strategy might provide tumor hormone responsiveness for a longer period. An animal model suggested that intermittent androgen deprivation (IAD) could prolong the duration of androgen dependence of hormone-sensitive tumors.[40]
Evidence (continuous vs. intermittent hormonal therapy):
- A systematic review of 15 randomized trials that compared continuous androgen deprivation versus IAD therapy for patients with advanced or recurrent prostate cancer found no significant difference in OS, which was reported in eight of the trials (HR, 1.02; 95% CI, 0.93–1.11); prostate–cancer-specific survival, reported in five of the trials (HR, 1.02; 95% CI, 0.87–1.19); or progression-free survival, reported in four of the trials (HR, 0.94; 95% CI, 0.84–1.05). The meta-analysis fulfilled prespecified criteria for noninferiority of OS (upper bound of 1.15 for the HRdeath, 1.15).[41][Level of evidence A1] However, of the 15 trials, all but one had an unclear or high risk of bias according to prespecified criteria.
- There was minimal difference in patient-reported QOL, but most trials found better physical and sexual functioning in patients in the IAD arms.
Radical prostatectomy with or without EBRT
Radical prostatectomy may be used with or without EBRT (in highly selected patients).[42] Because about 40% to 50% of men with clinically organ-confined disease are found to have pathological extension beyond the prostate capsule or surgical margins, the role of postprostatectomy adjuvant radiation therapy has been studied.
Evidence (radical prostatectomy with or without EBRT):
- In a randomized trial of 425 men with pathological T3, N0, M0 disease, postsurgical EBRT (60–64 Gy to the prostatic fossa over 30–32 fractions) was compared with observation.[43,44]
- After a median follow-up of about 12.5 years, OS was better in the radiation therapy arm; HRdeath, 0.72 (95% CI, 0.55–0.96; P = .023). The 10-year estimated survival rates were 74% in the radiation therapy arm and 66% in the control arm.
- The 10-year estimated metastasis-free survival rates were 73% and 65% (P = .016).[44][Level of evidence A1]
- Short-term complication rates were substantially higher in the radiation therapy group: overall complications were 23.8% versus 11.9%, rectal complications were 3.3% versus 0%, and urethral stricture was 17.8% versus 9.5%.
- The role of preoperative (neoadjuvant) hormonal therapy is not established.[45,46] Also, the morphologic changes induced by neoadjuvant androgen ablation may even complicate assessment of surgical margins and capsular involvement.[47]
Watchful waiting or active surveillance/active monitoring
Careful observation without further immediate treatment may be used in the treatment of stage III prostate cancer.[48,49]
Asymptomatic patients of advanced age or with concomitant illness may warrant consideration of careful observation without immediate active treatment.[50,51,52] Watch and wait, observation, expectant management, and active surveillance/active monitoring are terms indicating a strategy that does not employ immediate therapy with curative intent. For more information, see the Treatment Option Overview for Prostate Cancer section.
Treatment of Symptoms
Because many stage III patients have urinary symptoms, control of symptoms is an important consideration in treatment. The following modalities may be used to improve local control of disease and subsequent symptoms:
- Radiation therapy.
- Hormonal manipulation.
- Palliative surgery (transurethral resection of the prostate [TURP]).
- Interstitial implantation combined with EBRT.
- Alternative forms of radiation therapy (under clinical evaluation).
- Ultrasound-guided percutaneous cryosurgery (under clinical evaluation).
Radiation therapy
Radiation therapy may be used.[3,4,5,6] EBRT designed to decrease exposure of normal tissues using methods such as computed tomography–based 3-dimensional conformal radiation therapy treatment planning is under clinical evaluation.
Hormonal manipulation
Hormonal manipulations effectively used as initial therapy for prostate cancer include the following:
- Orchiectomy.
- Leuprolide or other LH-RH agonists (e.g., goserelin) in daily or depot preparations. These agents may be associated with tumor flare.
- Estrogens (diethylstilbestrol [DES] is no longer available in the United States).
- Nonsteroidal antiandrogens (e.g., flutamide, nilutamide, and bicalutamide) or steroidal antiandrogen (e.g., cyproterone acetate).
A meta-analysis of randomized trials comparing various hormonal monotherapies in men with stage III or stage IV prostate cancer (predominantly stage IV) came to the following conclusions:[53][Level of evidence A1]
- The OS at 2 years using any of the LH-RH agonists was similar to treatment with orchiectomy or 3 mg qd of DES (HR, 1.26; 95% CI, 0.92–1.39).
- Survival rates at 2 years were similar or worse with nonsteroidal antiandrogens compared with orchiectomy (HR, 1.22; 95% CI, 0.99–1.50).
- Treatment withdrawals, used as a surrogate for adverse effects, occurred less with LH-RH agonists (0%–4%) than with nonsteroidal antiandrogens (4%–10%).
Interstitial implantation combined with EBRT
Interstitial implantation combined with EBRT is being used in selected T3 patients, but little information is available.[54]
Alternative forms of radiation therapy
Alternative forms of radiation therapy are being employed in clinical trials.
- A randomized trial from the RTOG reported improved local control and survival with mixed-beam (neutron/photon) radiation therapy compared with standard photon radiation therapy.[55]
- A subsequent randomized study from the same group compared fast-neutron radiation therapy with standard photon radiation therapy. Local-regional control was improved with neutron treatment, but no difference in OS was seen, although follow-up was shorter in this trial. Fewer complications were seen with the use of a multileaf collimator.[56]
Proton-beam radiation therapy is also under investigation.[57]
Ultrasound-guided percutaneous cryosurgery
Ultrasound-guided percutaneous cryosurgery is under clinical evaluation.
Cryosurgery is a surgical technique under development that involves destruction of prostate cancer cells by intermittent freezing of the prostate with cryoprobes, followed by thawing.[58][Level of evidence C1]; [59]; [60][Level of evidence C3] Cryosurgery is less well established than standard prostatectomy, and long-term outcomes are not as well established as with prostatectomy or radiation therapy. Serious toxic effects include bladder outlet injury, urinary incontinence, sexual impotence, and rectal injury. The technique of cryosurgery is under development. Impotence is common. The frequency of other side effects and the probability of cancer control after 5 years of follow-up have varied among reporting centers, and series are small compared with surgery and radiation therapy.[59,60]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
-
Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 715–26.
-
Paulson DF: Management of prostate malignancy. In: deKernion JB, Paulson DF, eds.: Genitourinary Cancer Management. Lea and Febiger, 1987, pp 107-160.
-
Babaian RJ, Zagars GK, Ayala AG: Radiation therapy of stage C prostate cancer: significance of Gleason grade to survival. Semin Urol 8 (4): 225-31, 1990.
-
del Regato JA, Trailins AH, Pittman DD: Twenty years follow-up of patients with inoperable cancer of the prostate (stage C) treated by radiotherapy: report of a national cooperative study. Int J Radiat Oncol Biol Phys 26 (2): 197-201, 1993.
-
Pilepich MV, Johnson RJ, Perez CA, et al.: Prognostic significance of nodal involvement in locally advanced (stage C) carcinoma of prostate--RTOG experience. Urology 30 (6): 535-40, 1987.
-
Perez CA, Garcia D, Simpson JR, et al.: Factors influencing outcome of definitive radiotherapy for localized carcinoma of the prostate. Radiother Oncol 16 (1): 1-21, 1989.
-
Freeman JA, Lieskovsky G, Cook DW, et al.: Radical retropubic prostatectomy and postoperative adjuvant radiation for pathological stage C (PcN0) prostate cancer from 1976 to 1989: intermediate findings. J Urol 149 (5): 1029-34, 1993.
-
Kumar S, Shelley M, Harrison C, et al.: Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev (4): CD006019, 2006.
-
D'Amico AV, Chen MH, Renshaw AA, et al.: Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 299 (3): 289-95, 2008.
-
Bolla M, Van Tienhoven G, Warde P, et al.: External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 11 (11): 1066-73, 2010.
-
Seidenfeld J, Samson DJ, Aronson N, et al.: Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid Rep Technol Assess (Summ) (4): i-x, 1-246, I1-36, passim, 1999.
-
Roach M, Bae K, Speight J, et al.: Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 26 (4): 585-91, 2008.
-
Horwitz EM, Bae K, Hanks GE, et al.: Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26 (15): 2497-504, 2008.
-
Denham JW, Steigler A, Lamb DS, et al.: Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12 (5): 451-9, 2011.
-
Pilepich MV, Caplan R, Byhardt RW, et al.: Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol 15 (3): 1013-21, 1997.
-
Bolla M, Gonzalez D, Warde P, et al.: Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 337 (5): 295-300, 1997.
-
Seymore CH, el-Mahdi AM, Schellhammer PF: The effect of prior transurethral resection of the prostate on post radiation urethral strictures and bladder neck contractures. Int J Radiat Oncol Biol Phys 12 (9): 1597-600, 1986.
-
Roach M, DeSilvio M, Lawton C, et al.: Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 21 (10): 1904-11, 2003.
-
Pollack A: A call for more with a desire for less: pelvic radiotherapy with androgen deprivation in the treatment of prostate cancer. J Clin Oncol 21 (10): 1899-901, 2003.
-
Widmark A, Klepp O, Solberg A, et al.: Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 373 (9660): 301-8, 2009.
-
Warde P, Mason M, Ding K, et al.: Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 378 (9809): 2104-11, 2011.
-
Mason MD, Parulekar WR, Sydes MR, et al.: Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. J Clin Oncol 33 (19): 2143-50, 2015.
-
Brundage M, Sydes MR, Parulekar WR, et al.: Impact of Radiotherapy When Added to Androgen-Deprivation Therapy for Locally Advanced Prostate Cancer: Long-Term Quality-of-Life Outcomes From the NCIC CTG PR3/MRC PR07 Randomized Trial. J Clin Oncol 33 (19): 2151-7, 2015.
-
Pilepich MV, Winter K, Lawton CA, et al.: Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 61 (5): 1285-90, 2005.
-
Bolla M, Collette L, Blank L, et al.: Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360 (9327): 103-6, 2002.
-
Zagars GK, Johnson DE, von Eschenbach AC, et al.: Adjuvant estrogen following radiation therapy for stage C adenocarcinoma of the prostate: long-term results of a prospective randomized study. Int J Radiat Oncol Biol Phys 14 (6): 1085-91, 1988.
-
Granfors T, Modig H, Damber JE, et al.: Combined orchiectomy and external radiotherapy versus radiotherapy alone for nonmetastatic prostate cancer with or without pelvic lymph node involvement: a prospective randomized study. J Urol 159 (6): 2030-4, 1998.
-
Pilepich MV, Winter K, John MJ, et al.: Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 50 (5): 1243-52, 2001.
-
Horwitz EM, Winter K, Hanks GE, et al.: Subset analysis of RTOG 85-31 and 86-10 indicates an advantage for long-term vs. short-term adjuvant hormones for patients with locally advanced nonmetastatic prostate cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys 49 (4): 947-56, 2001.
-
Nabid A, Carrier N, Martin AG, et al.: Duration of Androgen Deprivation Therapy in High-risk Prostate Cancer: A Randomized Phase III Trial. Eur Urol 74 (4): 432-441, 2018.
-
Boustead G, Edwards SJ: Systematic review of early vs deferred hormonal treatment of locally advanced prostate cancer: a meta-analysis of randomized controlled trials. BJU Int 99 (6): 1383-9, 2007.
-
Pisansky TM, Hunt D, Gomella LG, et al.: Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol 33 (4): 332-9, 2015.
-
Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol 79 (2): 235-46, 1997.
-
James ND, de Bono JS, Spears MR, et al.: Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 377 (4): 338-351, 2017.
-
Kunath F, Grobe HR, Rücker G, et al.: Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev (6): CD009266, 2014.
-
Iversen P, Tyrrell CJ, Kaisary AV, et al.: Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J Urol 164 (5): 1579-82, 2000.
-
Kirk D: Immediate vs. deferred hormone treatment for prostate cancer: how safe is androgen deprivation? [Abstract] BJU Int 86 (Suppl 3): 218-58, 2000.
-
Studer UE, Hauri D, Hanselmann S, et al.: Immediate versus deferred hormonal treatment for patients with prostate cancer who are not suitable for curative local treatment: results of the randomized trial SAKK 08/88. J Clin Oncol 22 (20): 4109-18, 2004.
-
Studer UE, Whelan P, Albrecht W, et al.: Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol 24 (12): 1868-76, 2006.
-
Tombal B: Intermittent androgen deprivation therapy: conventional wisdom versus evidence. Eur Urol 55 (6): 1278-80, 2009.
-
Magnan S, Zarychanski R, Pilote L, et al.: Intermittent vs Continuous Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 1 (9): 1261-9, 2015.
-
Walsh PC, Jewett HJ: Radical surgery for prostatic cancer. Cancer 45 (7 Suppl): 1906-11, 1980.
-
Thompson IM, Tangen CM, Paradelo J, et al.: Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 296 (19): 2329-35, 2006.
-
Thompson IM, Tangen CM, Paradelo J, et al.: Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181 (3): 956-62, 2009.
-
Witjes WP, Schulman CC, Debruyne FM: Preliminary results of a prospective randomized study comparing radical prostatectomy versus radical prostatectomy associated with neoadjuvant hormonal combination therapy in T2-3 N0 M0 prostatic carcinoma. The European Study Group on Neoadjuvant Treatment of Prostate Cancer. Urology 49 (3A Suppl): 65-9, 1997.
-
Fair WR, Cookson MS, Stroumbakis N, et al.: The indications, rationale, and results of neoadjuvant androgen deprivation in the treatment of prostatic cancer: Memorial Sloan-Kettering Cancer Center results. Urology 49 (3A Suppl): 46-55, 1997.
-
Bazinet M, Zheng W, Bégin LR, et al.: Morphologic changes induced by neoadjuvant androgen ablation may result in underdetection of positive surgical margins and capsular involvement by prostatic adenocarcinoma. Urology 49 (5): 721-5, 1997.
-
Adolfsson J: Deferred treatment of low grade stage T3 prostate cancer without distant metastases. J Urol 149 (2): 326-8; discussion 328-9, 1993.
-
Stattin P, Holmberg E, Johansson JE, et al.: Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst 102 (13): 950-8, 2010.
-
Chodak GW, Thisted RA, Gerber GS, et al.: Results of conservative management of clinically localized prostate cancer. N Engl J Med 330 (4): 242-8, 1994.
-
Whitmore WF: Expectant management of clinically localized prostatic cancer. Semin Oncol 21 (5): 560-8, 1994.
-
Shappley WV, Kenfield SA, Kasperzyk JL, et al.: Prospective study of determinants and outcomes of deferred treatment or watchful waiting among men with prostate cancer in a nationwide cohort. J Clin Oncol 27 (30): 4980-5, 2009.
-
Seidenfeld J, Samson DJ, Hasselblad V, et al.: Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med 132 (7): 566-77, 2000.
-
Blasko JC, Grimm PD, Ragde H: Brachytherapy and Organ Preservation in the Management of Carcinoma of the Prostate. Semin Radiat Oncol 3 (4): 240-249, 1993.
-
Laramore GE, Krall JM, Thomas FJ, et al.: Fast neutron radiotherapy for locally advanced prostate cancer. Final report of Radiation Therapy Oncology Group randomized clinical trial. Am J Clin Oncol 16 (2): 164-7, 1993.
-
Russell KJ, Caplan RJ, Laramore GE, et al.: Photon versus fast neutron external beam radiotherapy in the treatment of locally advanced prostate cancer: results of a randomized prospective trial. Int J Radiat Oncol Biol Phys 28 (1): 47-54, 1994.
-
Shipley WU, Verhey LJ, Munzenrider JE, et al.: Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys 32 (1): 3-12, 1995.
-
Robinson JW, Saliken JC, Donnelly BJ, et al.: Quality-of-life outcomes for men treated with cryosurgery for localized prostate carcinoma. Cancer 86 (9): 1793-801, 1999.
-
Donnelly BJ, Saliken JC, Ernst DS, et al.: Prospective trial of cryosurgical ablation of the prostate: five-year results. Urology 60 (4): 645-9, 2002.
-
Aus G, Pileblad E, Hugosson J: Cryosurgical ablation of the prostate: 5-year follow-up of a prospective study. Eur Urol 42 (2): 133-8, 2002.
Treatment of Stage IV Prostate Cancer
Overview
Stage IV prostate cancer is defined by the American Joint Committee on Cancer's TNM (tumor, node, metastasis) classification system:[1]
Stage IVA
- Any T, N1, M0, any prostate-specific antigen (PSA), any Gleason.
Stage IVB
- Any T, N0, M1, any PSA, any Gleason.
Extraprostatic extension with microscopic bladder neck invasion (T4) is included with T3a.
Treatment selection depends on the following factors:
- Age.
- Coexisting medical illnesses.
- Symptoms.
- The presence of distant metastases (most often bone) or regional lymph node involvement only.
The most common symptoms originate from the urinary tract or from bone metastases. Palliation of symptoms from the urinary tract with transurethral resection of the prostate (TURP) or radiation therapy and palliation of symptoms from bone metastases with radiation therapy or hormonal therapy are an important part of the management of these patients. Bisphosphonates may also be used for the management of bone metastases.[2]
Treatment Options for Stage IV Prostate Cancer
Treatment options for patients with stage IV prostate cancer include the following:
- Hormonal manipulations.
- Hormonal manipulations with chemotherapy.
- Bisphosphonates.
- External-beam radiation therapy (EBRT) with or without hormonal therapy.
- Palliative radiation therapy.
- Palliative surgery with TURP.
- Watchful waiting or active surveillance/active monitoring.
- Radical prostatectomy with immediate orchiectomy (under clinical evaluation).
Hormonal manipulations
Hormonal treatment is the mainstay of therapy for metastatic prostate cancer. Cure is rarely, if ever, possible, but striking subjective or objective responses to treatment occur in most patients. The cornerstone of hormonal therapy for prostate cancer is medical or surgical castration to stop the production of testosterone by the testes. This is commonly referred to as androgen deprivation therapy (ADT) and can be achieved with bilateral orchiectomy or with administration of gonadotropin-releasing hormone (GnRH) agonists or antagonists. The most effective purely hormonal approach employs a combination of ADT and one of the following agents:
- Abiraterone acetate, an inhibitor of cytochrome P450c17, a critical enzyme in androgen biosynthesis.
- Apalutamide, an androgen receptor antagonist.
- Enzalutamide, an androgen receptor antagonist.
Randomized controlled trials have reported that combination therapy with any one of these drugs plus ADT results in longer overall survival than does ADT alone.
- In the randomized double-blind LATITUDE trial (NCT01715285), 1,199 men with high-risk metastatic castration-sensitive prostate cancer were given ADT plus either abiraterone acetate (1,000 mg PO qd) and prednisone (5 mg PO qd) or ADT plus abiraterone-prednisone placebos.[3] High-risk disease was defined as having at least two of the following three factors: Gleason score of 8 or higher, three or more bone lesions, or measurable visceral metastases.
- After a median follow-up of 30.4 months, the trial was stopped because of a clear overall survival (OS) benefit in the abiraterone study group: median survival not reached versus 34.7 months OS (hazard ratio [HR], 0.62; 95% confidence interval [CI], 0.51–0.76; P < .001).[3][Level of evidence A1]
- Abiraterone therapy was well tolerated, but there was an increase in the mineralocorticoid effects of grade 3 or 4 hypertension and hypokalemia compared with the placebo study group.
- A collection of patient-reported outcomes and Health-Related Quality of Life (HRQOL) data showed clinical benefits in pain progression, prostate cancer–related symptoms, fatigue, functional decline, and overall HRQOL in the abiraterone-acetate study group compared with the placebo group.[4][Level of evidence A3]
- In the randomized open-label STAMPEDE trial (NCT00268476), 1,917 men (about 95% newly diagnosed; about 50% had metastatic disease and about 50% had locally advanced or node-positive disease) were treated with ADT alone or ADT plus abiraterone acetate (1,000 mg PO qd) and prednisolone (5 mg PO qd).[5] Local radiation therapy was mandated after 6 to 9 months for men with node-negative nonmetastatic disease and optional for those with node-positive nonmetastatic disease. Hormone therapy was curtailed at 2 years or until progression. Radiation therapy was planned in about 40% of study participants.
- With a median follow-up of 40 months, the 3-year OS rate was 83% in the abiraterone study group compared with 76% in the ADT-only study group (HRdeath, 0.63; 95% CI, 0.52–0.76; P< .001).[5][Level of evidence A1] Although there was no clear evidence of heterogeneity in relative treatment differences in metastatic disease versus nonmetastatic disease, absolute differences were much smaller in men with nonmetastatic disease and not statistically significant, perhaps because of the short follow-up (HRdeath, 0.75; 95% CI, 0.49–1.18).
- The main additional differences in toxicity associated with abiraterone compared with ADT alone were hypertension (5% vs. 1%), mild increase in blood aminotransferase levels (6% vs. < 1%), and respiratory disorders (5% vs. 2%).
- In the randomized, controlled, double-blind phase III TITAN trial (NCT02489318), 1,052 men with metastatic, castration-sensitive prostate cancer were randomly assigned to receive ADT alone or ADT plus either apalutamide (240 mg PO qd) or placebo.[6]
- The 2-year OS rate was 82.4% in the apalutamide group and 73.5% in the placebo group (HR, 0.67; 95% CI, 0.51−0.89).
- Radiographic progression-free survival (PFS) was 68.2% in the apalutamide group and 47.5% in the placebo group (HR, 0.48; 95% CI, 0.39−0.60).
- Grade 3 or 4 adverse events were reported in 42.2% of patients in the apalutamide group and 40.8% of patients in the placebo group.
- Apalutamide has been associated with an increased risk of seizure, so men with a history of or predisposition to seizures were excluded from this trial.
- In the randomized, controlled, open-label phase III ENZAMET trial (NCT02446405), 1,125 men with castrate-sensitive prostate cancer were randomly assigned to receive ADT alone or ADT plus enzalutamide (160 mg PO qd).[7]
- The 3-year OS rate was 80% in the combined-therapy arm and 72% in the ADT monotherapy arm (HR, 0.67; 95% CI, 0.52−0.86).
- PSA PFS (HR, 0.39, P < .001) and clinical PFS (HR, 0.40; P < .001) were also longer in the combined-therapy arm.
- Serious adverse events were reported in 42% of patients in the enzalutamide arm compared with 34% in the monotherapy arm.
- Treatment was discontinued more frequently in the enzalutamide arm (33 vs. 14 events), and seizures and fatigue were more common in the enzalutamide arm: seven men (1%) had seizures in the enzalutamide arm versus none in the ADT-alone arm.
- Six percent of men in the combined-therapy arm reported grade 3 to 4 fatigue compared with 1% in the ADT-alone arm.
Hormonal manipulations effectively used as initial therapy for prostate cancer include the following:[8]
- Orchiectomy alone or with an androgen blocker as seen in the Southwest Oncology Group (SWOG-8894) trial.
- Luteinizing hormone-releasing hormone (LH-RH) agonists, such as leuprolide in daily or depot preparations. These agents may be associated with tumor flare when used alone; therefore, the initial concomitant use of antiandrogens should be considered in the presence of liver pain, ureteral obstruction, or impending spinal cord compression.[9,10,11,12][Level of evidence A1]
- Leuprolide plus flutamide;[13] however, the addition of an antiandrogen to leuprolide has not been clearly shown in a meta-analysis to improve survival.[14]
- Estrogens (diethylstilboestrol [DES], chlorotrianisene, ethinyl estradiol, conjugated estrogens-USP and DES-diphosphate). DES is no longer commercially available in the United States.
In some series, pretreatment levels of PSA were inversely correlated with progression-free duration in patients with metastatic prostate cancer who received hormonal therapy. After hormonal therapy is initiated, a PSA reduction to beneath a detectable level provides information regarding the duration of progression-free status; however, decreases in PSA of less than 80% may not be very predictive.[15]
Orchiectomy and estrogens yield similar results, and selection of one or the other depends on patient preference and the morbidity of expected side effects. Estrogens are associated with the development or exacerbation of cardiovascular disease, especially in high doses. DES at a dose of 1 mg qd is not associated with cardiovascular complications as frequent as those found at higher doses; however, the use of DES has decreased because of cardiovascular toxic effects.
The psychological implications of orchiectomy are objectionable to many patients, and many will choose an alternative therapy if effective.[16] Combined orchiectomy and estrogens are not indicated to be superior to either treatment administered alone.[17]
A large proportion of men experience hot flushes after bilateral orchiectomy or treatment with LH-RH agonists. These hot flashes can persist for years.[18] Varying levels of success in the management of these symptoms have been reported with DES, clonidine, cyproterone acetate, or medroxyprogesterone acetate.
After tumor progression on one form of hormonal manipulation, an objective tumor response to any other form is uncommon.[19] Some studies, however, suggest that withdrawal of flutamide (with or without aminoglutethimide administration) is associated with a decline in PSA and that one may need to monitor for this response before initiating new therapy.[20,21,22] Low-dose prednisone may palliate symptoms in about 33% of cases.[23] Newer hormonal approaches, such as inhibition of androgen receptors, have been shown to improve OS and quality of life (QOL) after tumor progression despite ADT. For more information, see the Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer section.
Immediate versus deferred hormonal therapy
Some patients may be asymptomatic and careful observation without further immediate therapy may be appropriate.
Evidence (immediate vs. deferred hormonal therapy):
- A meta-analysis of seven randomized controlled trials comparing early (adjuvant or neoadjuvant) with deferred hormonal treatment (LH-RH agonists and/or antiandrogens) in patients with locally advanced prostate cancer, whether treated with prostatectomy, radiation therapy, or watchful waiting or active surveillance/active monitoring, showed improved overall mortality with early treatment (relative risk, 0.86; 95% CI, 0.82–0.91).[24][Level of evidence A1]
- In a small, randomized trial of 98 men who underwent radical prostatectomy plus pelvic lymphadenectomy and were found to have nodal metastases (stage T1–2, N1, M0), immediate continuous hormonal therapy with the LH-RH agonist goserelin or with orchiectomy was compared with deferred therapy until documentation of disease progression.[25][Level of evidence A1];[26]
- After a median follow-up of 11.9 years, OS (P = .04) and prostate–cancer-specific survival (P = .004) were superior in the immediate adjuvant therapy arm.
- At 10 years, the survival rate in the immediate therapy arm was about 80% versus about 60% in the deferred therapy arm.[27]
- Another trial (RTOG-8531) with twice as many randomly assigned patients showed no difference in OS with early versus late hormonal manipulation.[28]
- Immediate hormonal therapy with goserelin or orchiectomy has also been compared with deferred hormonal therapy for clinical disease progression in a randomized trial (EORTC-30846) of men with regional lymph node involvement but no clinical evidence of metastases (any T, N+, M0). None of the 234 men had a prostatectomy or prostatic radiation therapy.[29][Level of evidence A1]
- After a median follow-up of 8.7 years, the HR for OS in the deferred versus immediate hormonal therapy arms was 1.23 (95% CI, 0.88–1.71).
- No statistically significant difference in OS between deferred and immediate hormonal therapy was found, but the trial was underpowered to detect small or modest differences.
- Immediate hormonal treatment (e.g., orchiectomy or LH-RH agonist) versus deferred treatment (e.g., watchful waiting with hormonal therapy at progression) was examined in a randomized study in men with locally advanced or asymptomatic metastatic prostate cancer.[30][Level of evidence A1]
- The initial results showed better OS and prostate–cancer-specific survival with immediate treatment.
- The incidence of pathological fractures, spinal cord compression, and ureteric obstruction were also lower in the immediate treatment arm.
- In another trial, 197 men with stage III or stage IV prostate cancer were randomly assigned to have a bilateral orchiectomy at diagnosis or at the time of symptomatic progression (or at the time of new metastases that were deemed likely to cause symptoms).[31][Level of evidence A1]
- After 12 years of follow-up, no statistically significant difference was observed in OS.
Luteinizing hormone-releasing hormone (LH-RH) agonists or antiandrogens
Approaches using LH-RH agonists or antiandrogens in patients with stage IV prostate cancer have produced response rates similar to other hormonal treatments.[9,32]
Evidence (LH-RH agonists or antiandrogens):
- In a randomized trial, the LH-RH agonist leuprolide (1 mg subcutaneously [SQ] qd) was as effective as DES (3 mg PO qd) in any T, any N, M1 patients, but caused less gynecomastia, nausea and vomiting, and thromboembolisms.[10]
- In other randomized studies, the depot LH-RH agonist goserelin was as effective as orchiectomy [11,33,34] or DES at a dose of 3 mg qd.[32] A depot preparation of leuprolide, which is therapeutically equivalent to daily leuprolide, is available as a monthly or 3-monthly depot.
- A systematic evidence review compared nonsteroidal antiandrogen monotherapy with surgical or medical castration from 11 randomized trials in 3,060 men with locally advanced, metastatic, or recurrent disease after local therapy.[35] Use of nonsteroidal antiandrogens as monotherapy decreased OS and increased the rate of clinical progression and treatment failure.[35][Level of evidence A1]
- A small randomized study comparing 1 mg DES PO tid with 250 mg of flutamide tid in patients with metastatic prostate cancer showed similar response rates with both regimens but superior survival with DES. More cardiovascular and/or thromboembolic toxic effects of borderline statistical significance were associated with DES treatment.[36][Level of evidence A1] A variety of combinations of hormonal therapy have been tested.
Maximal androgen blockade (MAB)
On the basis that the adrenal glands continue to produce androgens after surgical or medical castration, case series studies were performed in which antiandrogen therapy was added to castration. Promising results from the case series led to widespread use of the strategy, known as MAB or total androgen blockade. Subsequent randomized controlled trials, however, cast doubt on the efficacy of adding an antiandrogen to castration.
Evidence (MAB):
- In a large, randomized, controlled trial comparing treatment with bilateral orchiectomy plus either the antiandrogen flutamide or placebo, no difference in OS was reported.[37][Level of evidence A1]
- Although it has been suggested that MAB may improve the more subjective end point of response rate, prospectively assessed QOL was worse in the flutamide arm than in the placebo arm primarily because of more diarrhea and worse emotional function in the flutamide-treated group.[38][Level of evidence A3]
- A meta-analysis of 27 randomized trials of 8,275 patients comparing conventional surgical or medical castration with MAB—castration plus prolonged use of an antiandrogen such as flutamide, cyproterone acetate, or nilutamide—did not show a statistically significant improvement in survival associated with MAB.[14][Level of evidence A1]
When trials of androgen suppression versus androgen suppression plus either nilutamide or flutamide were examined in a subset analysis, the absolute survival rate at 5 years was better for the combined-therapy group (2.9% better, 95% CI, 0.3–5.5); however, when trials of androgen suppression versus androgen suppression plus cyproterone acetate were examined, the absolute survival trend at 5 years was worse for the combined-therapy group (2.8% worse, 95% CI, -7.6 to +2.0).[14]
- The Agency for Health Care Policy and Research (now the Agency for Healthcare Research and Quality) performed a systematic review of the available randomized, clinical trial evidence of single hormonal therapies and total androgen blockade performed by its Technology Evaluation Center, an evidence-based Practice Center of the Blue Cross and Blue Shield Association. A meta-analysis of randomized trials comparing various hormonal monotherapies in men with stage III or stage IV prostate cancer (predominantly stage IV) came to the following conclusions:[39][Level of evidence A1]
- OS at 2 years using any of the LH-RH agonists was similar to treatment with orchiectomy or 3 mg every day of DES (HR, 1.26; 95% CI, 0.92–1.39).
- Survival rates at 2 years were similar or worse with nonsteroidal antiandrogens compared with orchiectomy (HR, 1.22; 95% CI, 0.99–1.50).
- Treatment withdrawals, used as a surrogate for adverse effects, occurred less with LH-RH agonists (0%–4%) than with nonsteroidal antiandrogens (4%–10%).
Total androgen blockade was of no greater benefit than single hormonal therapy and with less patient tolerance. Also, the evidence was judged insufficient to determine whether men newly diagnosed with asymptomatic metastatic disease should have immediate androgen suppression therapy or should have therapy deferred until they have clinical signs or symptoms of progression.[40]
Continuous versus intermittent hormonal therapy
When used as the primary therapy for patients with stage III or stage IV prostate cancer, androgen suppression with hormonal therapy is often given continuously until there is disease progression. Another option is intermittent androgen suppression as a strategy to attain maximal tumor cytoreduction followed by a period without therapy to allow treatment-free periods. It was proposed that this strategy might provide tumor hormone responsiveness for a longer period. An animal model suggested that intermittent androgen deprivation (IAD) could prolong the duration of androgen dependence of hormone-sensitive tumors.[41] However, randomized controlled trials in humans have failed to support the hypothesis that IAD would delay the development of castration-resistant disease. If there are benefits from IAD, they appear to be in the realm of physical and sexual functioning.
Evidence (continuous vs. intermittent hormonal therapy):
- A systematic review of 15 randomized trials that compared continuous ADT versus IAD therapy for patients with advanced or recurrent prostate cancer found no significant difference in OS, which was reported in eight of the trials (HR, 1.02; 95% CI, 0.93–1.11); prostate cancer-specific survival, reported in five of the trials (HR, 1.02; 95% CI, 0.87–1.19); or PFS, reported in four of the trials (HR, 0.94; 95% CI, 0.84–1.05). The meta-analysis fulfilled prespecified criteria for noninferiority of OS (upper bound of 1.15 for the HRdeath, 1.15).[42][Level of evidence A1] However, of the 15 trials, all but one had an unclear or high risk of bias according to prespecified criteria.
- There was minimal difference in patient-reported QOL, but most trials found better physical and sexual functioning in patients in the IAD arms.
Hormonal manipulations with chemotherapy
The addition of chemotherapy has been shown in randomized trials to improve OS compared with ADT alone, with efficacy that appears to be comparable with hormonal therapy, which includes ADT plus abiraterone acetate. However, the two approaches have not been directly compared in a randomized study.
The addition of docetaxel has been tested in combination with long-term hormone therapy in the first-line management of metastatic prostate cancer and has been shown to improve results more than hormone therapy alone. A systematic evidence review and meta-analysis of randomized trials in hormone-sensitive metastatic prostate cancer summarizes these data.[43]
Evidence (hormonal manipulations with chemotherapy):
- In the analysis of three randomized trials (3,206 men), the HRdeath associated with the addition of docetaxel to standard of care was 0.77 (95% CI, 0.68–0.87; P < .0001), representing an absolute improvement of 9% in 4-year survival (95% CI, 5–14).[43][Level of evidence A1]
- In the CHAARTED trial (NCT00309985), 790 patients with metastatic, hormone-sensitive disease were randomly assigned to receive ADT with or without docetaxel (75 mg/m2 intravenously [IV] every 3 weeks for 6 cycles).[44,45] Previous adjuvant ADT was permissible if it lasted 12 months or less and progression had occurred within 12 months of completion. Patients were prospectively stratified as having a high- versus low-volume disease, with high volume defined as presence of visceral metastases or at least four bone lesions, with at least one lying outside the vertebral column or pelvis. About 65% of patients had high-volume disease by this definition.
- With a median follow-up of 53.7 months, median OS in the ADT-plus-docetaxel arm was 57.6 months and in the ADT-alone arm, it was 47.2 months (HRdeath, 0.72; 95% CI, 0.59–0.89; P = .0018).[45][Level of evidence A1]
- The survival advantage was observed only in patients with high-volume disease. In the group with high-volume disease, there was a clear improvement in median OS (61.2 months vs. 34.4 months) (HR, 0.63; 95% CI, 0.50–0.79; P < .001). However, there was no observed difference in survival in men with low-volume disease (median OS, 63.5 months vs. not reached) (HR, 1.04; 95% CI, 0.70–1.55; P = .86). The test for heterogeneity of efficacy was statistically significant (P = .033).
- Comparison of QOL between the two study groups, as measured by the Functional Assessment of Cancer Therapy-Prostate (FACT-P) scale, was not found to exceed the prospectively defined minimally important difference at any time point over the 12 months of planned assessment.[46]
Bisphosphonates
In addition to hormonal therapy, adjuvant treatment with bisphosphonates has been tested.[47]
Evidence (bisphosphonates):
- In MRC-PR05, 311 men with bone metastases who were starting or responding to standard hormonal therapy were randomly assigned to oral sodium clodronate (2,080 mg qd) or a matching placebo for up to 3 years.[47][Level of evidence A1]
- At a median follow-up of 11.5 years, OS was better in the clodronate arm: HRdeath, 0.77 (95% CI, 0.60–0.98; P = .032).
- Five- and 10-year survival rates were 30% and 17% in the clodronate arm versus 21% and 9% in the placebo arm.
- A parallel study (MRC-PR04) in men with locally advanced but nonmetastatic disease showed no benefit associated with clodronate.
- CALGB-90202 [NCT00079001] was a randomized controlled trial that compared zoledronic acid (4 mg IV every 4 weeks) with placebo in 645 men with androgen deprivation-sensitive prostate cancer that was metastatic to bone. Patients who progressed on hormone-therapy resistance received open-label, zoledronic acid.[48][Level of evidence B1]
- There was no difference between the two study arms in risk of the primary end point of time to skeletal-related events (defined as the need for palliative bone radiation, clinical fracture, spinal cord compression, bone surgery, or death from prostate cancer) after up to 7 years of follow-up.
- There were also no differences in PFS or OS.
- In another negative randomized trial (STAMPEDE [NCT00268476]), 1,245 men with locally advanced (M0) or metastatic (M1) prostate cancer, who were initiating long-term hormonal therapy, were randomly assigned in a 2:1:1 ratio to one of three arms: standard of care, celecoxib (400 mg bid for 1 year), and celecoxib plus zoledronic acid (4 mg IV for six 3-week cycles, then 4-week cycles for 2 years).[49]
- After a median follow-up of 69 months, there was no detectable improvement in survival associated with either celecoxib or celecoxib plus zoledronic acid.
- Although survival was better in patients with M disease who received celecoxib plus zoledronic acid than in patients with M1 disease who received the standard of care (HRdeath, 0.78; 95% CI, 0.62–0.98), a formal test for interaction with metastasis status was not statistically significant; therefore, the unexpected finding can only be considered hypothesis-generating.
Bisphosphonates and decreasing risk of bone metastases
Patients with locally advanced nonmetastatic disease (T2–T4, N0–N1, and M0) are at risk of developing bone metastases, and bisphosphonates are being studied as a strategy to decrease this risk. However, a placebo-controlled randomized trial (MRC-PR04) of a 5-year regimen of the first-generation bisphosphonate clodronate in high oral doses (2,080 mg qd) had no favorable impact on either time to symptomatic bone metastasis or survival.[50][Level of evidence A1]
External-beam radiation therapy (EBRT) with or without hormonal therapy
EBRT may be used for attempted cure in highly selected stage M0 patients.[51,52] Definitive radiation therapy should be delayed 4 to 6 weeks after TURP to reduce incidence of stricture.[53]
Hormonal therapy should be considered in addition to EBRT.[40,54]
Evidence (radiation therapy with or without hormonal therapy):
- The Blue Cross and Blue Shield Association Technology Evaluation Center, an evidence-based practice center of the Agency for Healthcare Research and Quality (AHRQ), performed a systematic review of the available randomized clinical trial evidence comparing radiation therapy with radiation therapy and prolonged androgen suppression.[40][Level of evidence A1] Some patients with bulky T2b tumors were included in the studied groups.
- The meta-analysis found a difference in 5-year OS in favor of radiation therapy plus continued androgen suppression using an LH-RH agonist or orchiectomy compared with radiation therapy alone (HR, 0.63; 95% CI, 0.48–0.83).
- In a randomized, prospective clinical trial, 18 months of androgen suppression with an LH-RH agonist appears to have provided results that were similar to 36 months with respect to OS and disease-specific survival.[55][Level of evidence A1] In the trial, 630 men with stage II to stage IVA cancer (clinical stage T3–T4, or PSA >20 ng/ml, or Gleason score >7) received 70 Gy of radiation in 35 fractions alone plus a total of either 18 or 36 months of goserelin acetate.
- With a median follow-up of 9.4 years, OS was nearly identical in each study arm (62% at 10 years; HRdeath, 1.02; 95% CI, 0.81–1.29, P = .8), as was prostate cancer–specific survival (HRprostate death, 0.95; 95% CI, 0.58–1.55, P = .8).
- Global quality of life was nearly identical on both study arms, but sexual activity and interest in sex was moderately better in the 18-month arm.[55][Level of evidence A3]
- The optimal duration of neoadjuvant hormonal therapy has been studied. In a randomized trial (TROG 96.01 [ACTRN12607000237482]) of 818 men with locally advanced (T2b, T2c, T3, and T4), nonmetastatic cancer treated with radiation therapy (i.e., 66 Gy in 2 Gy daily fractions to the prostate and seminal vesicles but not including regional nodes). Patients were randomly assigned to radiation therapy alone, 3 months of neoadjuvant androgen deprivation therapy (NADT) (goserelin 3.6 mg SQ each month plus flutamide 250 mg PO tid) for 2 months before and during radiation, or 6 months of NADT for 5 months before and during radiation.[54][Level of evidence A1]
- After a median follow-up of 10.6 years, there were no statistically significant differences between the radiation alone group and the radiation plus 3 months of NADT group.
- However, the 6-month NADT arm showed better prostate cancer-specific mortality and overall mortality than radiation alone; 10-year all-cause mortality 29.2% versus 42.5% (HR, 0.63; 95% CI, 0.48–0.83, P = .0008).
- The duration of neoadjuvant hormonal therapy was tested in another trial (RTOG-9910 [NCT00005044]) of 1,489 eligible men with intermediate-risk prostate cancer (T1b–4, Gleason score 2–6, and PSA >10 but ≤100 ng/mL; T1b–4, Gleason score 7, and PSA <20; or T1b–1c, Gleason score 8–10, and PSA <20) and no evidence of metastases. The men were randomly assigned to receive short-course neoadjuvant–androgen suppression (an LH-RH agonist plus bicalutamide or flutamide for 8 weeks before and 8 weeks during radiation therapy) or long-course neoadjuvant-androgen suppression (28 weeks before and 8 weeks during radiation therapy). Both groups received 70.2 Gy radiation in 39 daily fractions to the prostate and 46.8 Gy to the iliac lymph nodes.[56][Level of evidence A1]
- After a median of 9.4 years, 10-year prostate specific mortality, the primary end point, was low in both study arms: 5% versus 4% (HR, 0.81; 95% CI, 0.48–1.39).[56][Level of evidence A1]
- No statistically significant differences in overall mortality or in locoregional disease progression were found.[56][Level of evidence A1]
- There was also no apparent differential effect of androgen suppression duration among any of the risk-group subsets.
Palliative radiation therapy
A single fraction of 8 Gy has been shown to have similar benefits on bone pain relief and QOL as multiple fractions (3 Gy × 10) as was evidenced in the RTOG-9714 trial (NCT00003162).[57]; [58][Level of evidence A3] For more information, see Cancer Pain.
Palliative surgery with transurethral resection of the prostate (TURP)
Transurethral resection of the prostate may be useful in relieving urinary obstruction as part of palliative care in advanced prostate cancer.
Watchful waiting or active surveillance/active monitoring
Careful observation without further immediate treatment (in selected asymptomatic patients).[59]
Radical prostatectomy with immediate orchiectomy
An uncontrolled, retrospective review of a large series of patients with any T, N1–3, M0 disease treated at the Mayo Clinic with concurrent radical prostatectomy and orchiectomy was associated with intervals to local and distant progression; however, increase in OS has not been demonstrated.[60] Patient selection factors make such study designs difficult to interpret.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
-
Prostate. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 715–26.
-
Dearnaley DP, Sydes MR, Mason MD, et al.: A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 95 (17): 1300-11, 2003.
-
Fizazi K, Tran N, Fein L, et al.: Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 377 (4): 352-360, 2017.
-
Chi KN, Protheroe A, Rodríguez-Antolín A, et al.: Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol 19 (2): 194-206, 2018.
-
James ND, de Bono JS, Spears MR, et al.: Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 377 (4): 338-351, 2017.
-
Chi KN, Agarwal N, Bjartell A, et al.: Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 381 (1): 13-24, 2019.
-
Davis ID, Martin AJ, Stockler MR, et al.: Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 381 (2): 121-131, 2019.
-
Scott WW, Menon M, Walsh PC: Hormonal therapy of prostatic cancer. Cancer 45 (7 Suppl): 1929-36, 1980.
-
Parmar H, Edwards L, Phillips RH, et al.: Orchiectomy versus long-acting D-Trp-6-LHRH in advanced prostatic cancer. Br J Urol 59 (3): 248-54, 1987.
-
Leuprolide versus diethylstilbestrol for metastatic prostate cancer. The Leuprolide Study Group. N Engl J Med 311 (20): 1281-6, 1984.
-
Peeling WB: Phase III studies to compare goserelin (Zoladex) with orchiectomy and with diethylstilbestrol in treatment of prostatic carcinoma. Urology 33 (5 Suppl): 45-52, 1989.
-
Sharifi R, Soloway M: Clinical study of leuprolide depot formulation in the treatment of advanced prostate cancer.The Leuprolide Study Group. J Urol 143 (1): 68-71, 1990.
-
Crawford ED, Eisenberger MA, McLeod DG, et al.: A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 321 (7): 419-24, 1989.
-
Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet 355 (9214): 1491-8, 2000.
-
Matzkin H, Eber P, Todd B, et al.: Prognostic significance of changes in prostate-specific markers after endocrine treatment of stage D2 prostatic cancer. Cancer 70 (9): 2302-9, 1992.
-
Cassileth BR, Soloway MS, Vogelzang NJ, et al.: Patients' choice of treatment in stage D prostate cancer. Urology 33 (5 Suppl): 57-62, 1989.
-
Byar DP: Proceedings: The Veterans Administration Cooperative Urological Research Group's studies of cancer of the prostate. Cancer 32 (5): 1126-30, 1973.
-
Karling P, Hammar M, Varenhorst E: Prevalence and duration of hot flushes after surgical or medical castration in men with prostatic carcinoma. J Urol 152 (4): 1170-3, 1994.
-
Small EJ, Vogelzang NJ: Second-line hormonal therapy for advanced prostate cancer: a shifting paradigm. J Clin Oncol 15 (1): 382-8, 1997.
-
Scher HI, Kelly WK: Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J Clin Oncol 11 (8): 1566-72, 1993.
-
Sartor O, Cooper M, Weinberger M, et al.: Surprising activity of flutamide withdrawal, when combined with aminoglutethimide, in treatment of "hormone-refractory" prostate cancer. J Natl Cancer Inst 86 (3): 222-7, 1994.
-
Small EJ, Srinivas S: The antiandrogen withdrawal syndrome. Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer 76 (8): 1428-34, 1995.
-
Tannock I, Gospodarowicz M, Meakin W, et al.: Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 7 (5): 590-7, 1989.
-
Boustead G, Edwards SJ: Systematic review of early vs deferred hormonal treatment of locally advanced prostate cancer: a meta-analysis of randomized controlled trials. BJU Int 99 (6): 1383-9, 2007.
-
Messing EM, Manola J, Sarosdy M, et al.: Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 341 (24): 1781-8, 1999.
-
Eisenberger MA, Walsh PC: Early androgen deprivation for prostate cancer? N Engl J Med 341 (24): 1837-8, 1999.
-
Messing EM, Manola J, Yao J, et al.: Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 7 (6): 472-9, 2006.
-
Lawton CA, Winter K, Grignon D, et al.: Androgen suppression plus radiation versus radiation alone for patients with stage D1/pathologic node-positive adenocarcinoma of the prostate: updated results based on national prospective randomized trial Radiation Therapy Oncology Group 85-31. J Clin Oncol 23 (4): 800-7, 2005.
-
Schröder FH, Kurth KH, Fosså SD, et al.: Early versus delayed endocrine treatment of pN1-3 M0 prostate cancer without local treatment of the primary tumor: results of European Organisation for the Research and Treatment of Cancer 30846--a phase III study. J Urol 172 (3): 923-7, 2004.
-
Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol 79 (2): 235-46, 1997.
-
Studer UE, Hauri D, Hanselmann S, et al.: Immediate versus deferred hormonal treatment for patients with prostate cancer who are not suitable for curative local treatment: results of the randomized trial SAKK 08/88. J Clin Oncol 22 (20): 4109-18, 2004.
-
Waymont B, Lynch TH, Dunn JA, et al.: Phase III randomised study of zoladex versus stilboestrol in the treatment of advanced prostate cancer. Br J Urol 69 (6): 614-20, 1992.
-
Vogelzang NJ, Chodak GW, Soloway MS, et al.: Goserelin versus orchiectomy in the treatment of advanced prostate cancer: final results of a randomized trial. Zoladex Prostate Study Group. Urology 46 (2): 220-6, 1995.
-
Kaisary AV, Tyrrell CJ, Peeling WB, et al.: Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br J Urol 67 (5): 502-8, 1991.
-
Kunath F, Grobe HR, Rücker G, et al.: Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev (6): CD009266, 2014.
-
Chang A, Yeap B, Davis T, et al.: Double-blind, randomized study of primary hormonal treatment of stage D2 prostate carcinoma: flutamide versus diethylstilbestrol. J Clin Oncol 14 (8): 2250-7, 1996.
-
Eisenberger MA, Blumenstein BA, Crawford ED, et al.: Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 339 (15): 1036-42, 1998.
-
Moinpour CM, Savage MJ, Troxel A, et al.: Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst 90 (20): 1537-44, 1998.
-
Seidenfeld J, Samson DJ, Hasselblad V, et al.: Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med 132 (7): 566-77, 2000.
-
Seidenfeld J, Samson DJ, Aronson N, et al.: Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid Rep Technol Assess (Summ) (4): i-x, 1-246, I1-36, passim, 1999.
-
Calais da Silva FE, Bono AV, Whelan P, et al.: Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol 55 (6): 1269-77, 2009.
-
Magnan S, Zarychanski R, Pilote L, et al.: Intermittent vs Continuous Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 1 (9): 1261-9, 2015.
-
Vale CL, Burdett S, Rydzewska LH, et al.: Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol 17 (2): 243-56, 2016.
-
Sweeney CJ, Chen YH, Carducci M, et al.: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 373 (8): 737-46, 2015.
-
Kyriakopoulos CE, Chen YH, Carducci MA, et al.: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol 36 (11): 1080-1087, 2018.
-
Morgans AK, Chen YH, Sweeney CJ, et al.: Quality of Life During Treatment With Chemohormonal Therapy: Analysis of E3805 Chemohormonal Androgen Ablation Randomized Trial in Prostate Cancer. J Clin Oncol 36 (11): 1088-1095, 2018.
-
Dearnaley DP, Mason MD, Parmar MK, et al.: Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol 10 (9): 872-6, 2009.
-
Smith MR, Halabi S, Ryan CJ, et al.: Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol 32 (11): 1143-50, 2014.
-
Mason MD, Clarke NW, James ND, et al.: Adding Celecoxib With or Without Zoledronic Acid for Hormone-Naïve Prostate Cancer: Long-Term Survival Results From an Adaptive, Multiarm, Multistage, Platform, Randomized Controlled Trial. J Clin Oncol 35 (14): 1530-1541, 2017.
-
Mason MD, Sydes MR, Glaholm J, et al.: Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873). J Natl Cancer Inst 99 (10): 765-76, 2007.
-
Bagshaw MA: External radiation therapy of carcinoma of the prostate. Cancer 45 (7 Suppl): 1912-21, 1980.
-
Ploysongsang S, Aron BS, Shehata WM, et al.: Comparison of whole pelvis versus small-field radiation therapy for carcinoma of prostate. Urology 27 (1): 10-6, 1986.
-
Seymore CH, el-Mahdi AM, Schellhammer PF: The effect of prior transurethral resection of the prostate on post radiation urethral strictures and bladder neck contractures. Int J Radiat Oncol Biol Phys 12 (9): 1597-600, 1986.
-
Denham JW, Steigler A, Lamb DS, et al.: Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12 (5): 451-9, 2011.
-
Nabid A, Carrier N, Martin AG, et al.: Duration of Androgen Deprivation Therapy in High-risk Prostate Cancer: A Randomized Phase III Trial. Eur Urol 74 (4): 432-441, 2018.
-
Pisansky TM, Hunt D, Gomella LG, et al.: Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol 33 (4): 332-9, 2015.
-
Kaasa S, Brenne E, Lund JA, et al.: Prospective randomised multicenter trial on single fraction radiotherapy (8 Gy x 1) versus multiple fractions (3 Gy x 10) in the treatment of painful bone metastases. Radiother Oncol 79 (3): 278-84, 2006.
-
Chow E, Harris K, Fan G, et al.: Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 25 (11): 1423-36, 2007.
-
Stattin P, Holmberg E, Johansson JE, et al.: Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst 102 (13): 950-8, 2010.
-
Zincke H: Extended experience with surgical treatment of stage D1 adenocarcinoma of prostate. Significant influences of immediate adjuvant hormonal treatment (orchiectomy) on outcome. Urology 33 (5 Suppl): 27-36, 1989.
Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
Overview
In recurrent hormone-sensitive or hormone-resistant prostate cancer, the selection of further treatment depends on many factors, including the following:
- Previous treatment.
- Site of recurrence.
- Coexistent illnesses.
- Individual patient considerations.
Definitive radiation therapy can be given to patients with disease that fails only locally after prostatectomy.[1,2,3,4] A randomized trial (RTOG-9601 [NCT00002874]) has shown improved overall survival (OS) and prostate–cancer-specific survival with the addition of high-dose bicalutamide to radiation therapy compared with radiation therapy alone in men with locally recurrent prostate cancer after radical prostatectomy.[5]
- In the trial, 760 men who were initially treated with radical prostatectomy for tumor stage T2 or T3, and who had a detectable prostate-specific antigen (PSA) level of 0.2 to 4.0 ng/mL, but no evidence of metastases, were randomly assigned to receive radiation (64.8 Gy over 36 fractions) and either bicalutamide (150 mg PO qd) or placebo for 24 months. The median interval from surgery to PSA detectability was 1.4 years and from surgery to randomization was 2.1 years. Median follow-up was 13 years.
- Actuarial OS at 12 years was 76.3% in the bicalutamide group versus 71.3% in the placebo group (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.59–0.99; P = .04).[5][Level of evidence A1]
- Prostate–cancer-specific mortality at 12 years was 5.8% (bicalutamide) versus 13.4% (placebo), (HR, 0.49; 95% CI, 0.32–0.74; P < .001).[5][Level of evidence A1]
- Most treatment-related toxicities were similar between the two groups, except for gynecomastia, which occurred in 69.7% of the men on bicalutamide versus 10.9% of those on placebo. This side effect may be mitigated by prophylactic breast irradiation, which was not used in this study because of the double-blinded design.
Some patients with a local recurrence after definitive radiation therapy can undergo salvage prostatectomy;[6] however, only about 10% of patients treated initially with radiation therapy will have local relapse only. In these patients, prolonged disease control is often possible with hormonal therapy, with median cancer-specific survival of 6 years after local failure.[7]
Cryosurgical ablation of recurrence after radiation therapy is associated frequently with a high complication rate. This technique is still undergoing clinical evaluation.[8]
Hormonal therapy is used to manage most relapsing patients with disseminated disease who initially received locoregional therapy with surgery or radiation therapy. For more information, see the Treatment Options for Stage IV Prostate Cancer section.
Immediate Versus Deferred Hormonal Therapy
For more information on the use of immediate hormonal therapy (bicalutamide or luteinizing hormone-releasing hormone [LH-RH] agonists) plus radiation in patients with locally recurrent prostate cancer after radical prostatectomy, see the Treatment Option Overview for Prostate Cancer section.
PSA is often used to monitor patients after initial therapy with curative intent, and elevated or rising PSA is a common trigger for additional therapy even in asymptomatic men. Despite how common the situation is, it is not clear whether additional treatments given because of rising PSA in asymptomatic men with prostate cancer increase OS. The quality of evidence is limited.
- After radical prostatectomy, detectable PSA levels identify patients at elevated risk of local treatment failure or metastatic disease;[9] however, a substantial proportion of patients with elevated or rising PSA levels after initial therapy with curative intent may remain clinically free of symptoms for extended periods.[10] In a retrospective analysis of nearly 2,000 men who had undergone radical prostatectomy with curative intent and who were followed for a mean of 5.3 years, 315 men (15%) demonstrated an abnormal PSA of 0.2 ng/mL or higher, which is evidence of biochemical recurrence.[11]
- Of these 315 men, 103 men (34%) developed clinical evidence of recurrence.
- The median time to development of clinical metastasis after biochemical recurrence was 8 years.
- After the men developed metastatic disease, the median time to death was an additional 5 years.
- After radiation therapy with curative intent, persistently elevated or rising PSA may be a prognostic factor for clinical disease recurrence. However, reported case series have used a variety of definitions of PSA failure. Criteria have been developed by the American Society for Therapeutic Radiology and Oncology Consensus Panel.[12,13] The implication of the various definitions of PSA failure for OS is not known, and as in the surgical series, many biochemical relapses (rising PSA alone) may not be clinically manifested in patients treated with radiation therapy.[14,15]
- A randomized trial (PMCC-VCOG-PR-0103 [NCT00110162]) of androgen deprivation therapy (ADT) in men who received curative therapy but had a rising PSA, provided some evidence of improved OS associated with immediate versus delayed therapy.[16] The study had important shortcomings.
- Two groups of men were randomly assigned to open-label, immediate-versus-delayed (at least 2-year delay) ADT:
- Group 1 included men who had a PSA relapse after curative therapy (89% of the study population).
- Group 2 included asymptomatic men who were considered unsuitable for curative treatment because of age, comorbidity, or locally advanced disease (11% of the study population).
Planned accrual was 750 patients, but because of slow accrual, the trial closed at 293 patients.
- In groups 1 and 2 combined, with a median follow-up of 5 years, the 5-year OS rate was 86.4% in the delayed ADT study arm versus 91.2% in the immediate ADT study arm (log-rank P = .047).[16][Level of evidence A1] After full adjustment for baseline characteristics, the HR for OS was 0.54 (95% CI, 0.27–1.06; P = .074).
- For group 1 only (those with PSA relapse after curative therapy, N = 261), the estimated 5-year survival rate was 78.2% versus 84.3% with delayed-versus-immediate ADT (log-rank P = .10; fully adjusted HR, 0.59; 95% CI, 0.26–1.30, P = .19).
- Toxicity was greater in the immediate ADT study arm compared with delayed therapy. Serious (grade 4) adverse events were reported in 42% of patients in the immediate ADT study arm versus 31% of patients in the delayed therapy arm. Quality of life (QOL) fell by 6.1% (considered a small but clinically important drop) with immediate ADT versus 3% with delayed ADT (considered a trivial drop); this was not a statistically significant difference (P = .14).[16] Sexual activity was lower and hormone-related symptoms (hot flashes and sore or enlarged nipples) were clinically and statistically significantly worse in the immediate ADT arm compared with the delayed therapy arm.[17]
Hormonal Therapy for Recurring Disease
Continuous versus intermittent hormonal therapy
Most men who are treated for recurrence after initial local therapy are asymptomatic, and the recurrence is detected by a rising PSA. It is possible that intermittent androgen deprivation (IAD) therapy can be used as an alternative to continuous ADT (CAD) to improve QOL and decrease the amount of time during which the patient experiences the side effects of hormonal therapy, without decreasing the survival rate.
- This important clinical question was addressed in a noninferiority-designed, randomized, controlled trial with 1,386 men who had rising PSA levels (>3 ng/mL, with serum testosterone >5 nmol/L) more than 1 year after primary or salvage radiation therapy for localized prostate cancer.[18][Levels of evidence A1 and A3]
- The ADT arm consisted of 8-month treatment cycles with an LH-RH agonist (combined with a nonsteroidal antiandrogen for at least the first 4 weeks) that was reinstituted if the PSA level exceeded 10 ng/mL. The study was powered to detect (with 95% confidence) an 8% lower OS rate in the IAD group compared with the CAD group at 7 years.
- After a median follow-up of 6.9 years (maximum follow-up, 11.2 years), OS in the two groups was nearly identical, and the study was stopped (median survival, 8.8 vs. 9.1 years; HRdeath, 1.02; 95% CI, 0.86–1.21). This fulfilled the prospective criterion of noninferiority.
- In a retrospective analysis, prostate–cancer-specific mortality was also similar in the two arms (HR, 1.18; 95% CI, 0.90–1.55; P = 0.24). In addition, IAD was statistically significantly better than CAD in several QOL domains, such as hot flashes, desire for sexual activity, and urinary symptoms. Patients on the IAD study arm received a median of 15.4 months of treatment versus 43.9 months on the CAD arm.
- The study did not address the unanswered question about whether the initiation of any ADT for an elevated PSA after initial local therapy extends survival compared with delay until clinically symptomatic progression. Of note, 59% of all deaths were unrelated to prostate cancer, and 14% of all patients died of prostate cancer.
- A systematic review of 15 randomized trials that compared CAD versus IAD therapy for patients with advanced or recurrent prostate cancer found no significant difference in OS, which was reported in eight of the trials (HR, 1.02; 95% CI, 0.93–1.11); prostate–cancer-specific survival, reported in five of the trials (HR, 1.02; 95% CI, 0.87–1.19); or progression-free survival (PFS), reported in four of the trials (HR, 0.94; 95% CI, 0.84–1.05). The meta-analysis fulfilled prespecified criteria for noninferiority of OS (upper bound of 1.15 for the HR of 1.15).[19][Level of evidence A1] However, of the 15 trials, all but one had an unclear or high risk of bias according to prespecified criteria.
- There was minimal difference in patient-reported QOL, but most trials found better physical and sexual functioning in patients in the IAD arms.
Nonsteroidal antiandrogen therapy with or without androgen deprivation therapy
Enzalutamide was tested with or without leuprolide in patients with clinically nonmetastatic, hormone–sensitive prostate cancer with high-risk biochemical recurrence (defined as a PSA doubling time ≤9 months and a PSA >2 ng/mL above nadir after radiation therapy, or PSA >1 ng/mL after radical prostatectomy with or without postoperative radiation therapy; M0 by conventional imaging). In practice, it is recommended that these patients undergo staging with prostate-specific membrane antigen positron emission tomography–computed tomography.
- The phase III EMBARK trial (NCT02319837) included 1,068 men. Patients had received prior definitive therapy with radical prostatectomy and/or radiation therapy with curative intent, had a rapidly rising PSA, and were not candidates for salvage pelvic-directed therapy. Patients were randomly assigned in a 1:1:1 ratio to receive blinded enzalutamide (160 mg PO qd) with leuprolide, blinded placebo (PO qd) plus leuprolide, or open-label single-agent enzalutamide (160 mg PO qd).[20]
- After a follow-up of 60.7 months, the enzalutamide-leuprolide combination was superior to leuprolide monotherapy for the primary end point of 5-year metastasis-free survival (87.3% vs. 71.4%; HR, 0.42; 95% CI, 0.30–0.61; P < .001). After a follow-up of 60.7 months, enzalutamide monotherapy was superior to leuprolide monotherapy for a key secondary end point of 5-year metastasis-free survival (80.0% vs. 71.4%; HR, 0.63; 95% CI, 0.46–0.87; P < .005).[20][Level of evidence B1]
- OS data were not mature, but at the time of the report, 12% of deaths were in the overall population, 3.4% were in the enzalutamide-leuprolide combination group, 6.1% were in the leuprolide monotherapy group, and 5.4% were in the enzalutamide monotherapy group.
- Treatment suspension in all arms occurred at 36 weeks if PSA reached undetectable levels (<0.2 ng/mL). Treatment could be restarted per assigned treatment arms when the PSA increased to >2.0 ng/mL for patients who had a prior prostatectomy, or >5.0 ng/mL for patients who had prior primary radiation therapy. In the enzalutamide-leuprolide combination group, 90.9% of patients had treatment suspended for a median of 20.2 months. In the leuprolide monotherapy arm, 67.8% of patients had treatment suspended for a median of 16.8 months. In the enzalutamide monotherapy arm, 85.9% of patients had treatment suspended for a median of 11.1 months.
- There was no substantial between-group differences in QOL measures.
- No new safety signals were reported.
- Grade 3 or higher toxicities for any adverse event were 46.5% in the enzalutamide-leuprolide combination group, 42.7% in the leuprolide monotherapy group, and 50.0% in the enzalutamide monotherapy group.
- The most common adverse events in the combination group and leuprolide monotherapy group were hot flashes and fatigue. The most common adverse events in the enzalutamide monotherapy group were gynecomastia, hot flashes, and fatigue.
- Adverse events of special interest included fractures, cognitive and memory impairment, and seizures. Fractures occurred in 18.4% of patients in the combination group, 13.6% of patients in the leuprolide monotherapy group, and 11% of the patients in the enzalutamide monotherapy group. Cognitive and memory impairment occurred in 15% of patients in the combination group, 6.5% of patients in the leuprolide monotherapy group, and 14.1% of patients in the enzalutamide monotherapy group. Seizures occurred in 1.1% of patients in the combination group, 0% of patients in the leuprolide monotherapy group, and 0.8% of patients in the enzalutamide monotherapy group.
Nonsteroidal antiandrogen monotherapy versus surgical or medical castration
A systematic evidence review compared nonsteroidal antiandrogen monotherapy with surgical or medical castration from 11 randomized trials in 3,060 men with locally advanced, metastatic, or recurrent disease after local therapy.[21] The use of nonsteroidal antiandrogens as monotherapy decreased OS and increased the rate of clinical progression and treatment failure.[21][Level of evidence A1]
Hormonal approaches
As noted above, studies have shown that chemotherapy with docetaxel or cabazitaxel and immunotherapy with sipuleucel-T can prolong OS in patients with hormone-sensitive or hormone-resistant metastatic prostate cancer. Nevertheless, hormonal therapy has also been shown to improve survival even in men who have progressed after other forms of hormonal therapy as well as chemotherapy. Some forms of hormonal therapy are effective in the management of metastatic hormone–refractory prostate cancer.
Because there are no head-to-head comparisons, there are no trials to help decide which of these agents should be used first or in what sequence they should be used.
Even among patients with metastatic hormone-refractory prostate cancer, some heterogeneity is found in prognosis and in retained hormone sensitivity. In such patients who have symptomatic bone disease, several factors are associated with worsened prognosis: poor performance status, elevated alkaline phosphatase, abnormal serum creatinine, and short (<1 year) previous response to hormonal therapy.[22] The absolute level of PSA at the initiation of therapy in relapsed or hormone-refractory patients has not shown prognostic significance.[23]
Some patients whose disease has progressed on combined androgen blockade can respond to a variety of second-line hormonal therapies. Aminoglutethimide, hydrocortisone, flutamide withdrawal, progesterone, ketoconazole, and combinations of these therapies have produced PSA responses in 14% to 60% of patients treated and have also produced clinical responses of 0% to 25% when assessed. The duration of these PSA responses has ranged from 2 to 4 months.[24] Survival rates are similar whether ketoconazole plus hydrocortisone is initiated at the same time as antiandrogen (e.g., flutamide, bicalutamide, or nilutamide) withdrawal or when PSA has risen after an initial trial of antiandrogen withdrawal, as seen in the CLB-9583 trial (NCT00002760), for example.[25][Level of evidence A1] There are conflicting data on whether PSA changes in men undergoing chemotherapy are predictive of survival.[23,26]
Patients treated with either luteinizing-hormone agonists or estrogens as primary therapy are generally maintained with castrate levels of testosterone. One study from the Eastern Cooperative Oncology Group (ECOG) showed that a superior survival resulted when patients were maintained on primary androgen deprivation;[9] however, another study from SWOG (formerly the Southwest Oncology Group) did not show an advantage to continued androgen blockade.[27]
Evidence (hormonal approaches for castration-resistant progressive disease with no previous chemotherapy):
- Abiraterone acetate is an inhibitor of androgen biosynthesis that works by blocking cytochrome P450c17 (CYP17). Abiraterone has mineralocorticoid effects, producing an increased incidence of fluid retention and edema, hypokalemia, hypertension, and cardiac dysfunction. In addition, abiraterone is associated with hepatotoxicity.[28] However, compared with other therapies, abiraterone toxicities are mild. In a double-blinded placebo-controlled trial, 1,088 men with progressing hormone refractory disease (serum testosterone <50 ng per deciliter on previous antiandrogen therapy), no previous chemotherapy, and ECOG performance status (PS) 0 to 1 were given prednisone (5 mg PO bid) plus either abiraterone acetate (1,000 mg PO qd) or placebo.[29,30][Level of evidence A1] The coprimary end points were radiological PFS and OS. Four sequential analyses were planned.
- At the second interim analysis, after a median follow-up of 22.2 months, the study was stopped and unblinded because of aggregate efficacy and safety as assessed by the data monitoring committee. At that point, the radiological PFS had reached the prespecified stopping boundary in favor of abiraterone (median PFS, 16.5 months vs. 8.3 months; HR, 0.53; 95% CI, 0.45–0.62; P < .001).
- At the fourth (and final) analysis with a median follow-up of 49.2 months (maximum 60 months), 65% had died in the abiraterone-acetate study arm and 71% had died in the placebo study arm (HR, 0.81; 95% CI, 0.70–0.93; P = .033). Median OS was 34.7 versus 30.3 months.[30][Level of evidence A1]
- Median time to health-related QOL deterioration was long in the abiraterone study arm, as assessed by the Functional Assessment of Cancer Therapy-Prostate Version 4 (FACT-P) total score (12.7 months vs. 8.3 months; HR, 0.78; 95% CI, 0.66-0.92; P = .003) and by the prostate–cancer-specific subscale (11.1 months vs. 5.8 months; HR, 0.70; 95% CI, 0.60–0.83; P < .0001).[31][Level of evidence A3]
- In addition, patients in the abiraterone study group had statistically significant longer median times to opiate use for pain, initiation of cytotoxic chemotherapy, decline in PS, and PSA progression.[29,31][Levels of evidence A3 and B1]
- Enzalutamide, an androgen receptor antagonist, has been shown to increase OS and QOL in men with metastatic prostate cancer that has progressed despite ADT. In the PREVAIL study (NCT01212991), 1,717 asymptomatic or mildly symptomatic men with recurrent metastatic prostate cancer despite ADT were randomly assigned to receive enzalutamide (160 mg PO qd) versus placebo.[32,33,34][Levels of evidence A1 and A3]
- After a median follow-up of 22 months, the study was stopped because of an OS benefit in the enzalutamide study arm (HR, 0.72; 95% CI, 0.6–0.84; P < .001). The proportion of men who had died was 28% versus 35%, and the median OS was 32.4 versus 30.2 months.
- Median time until decline in global QOL, measured by the FACT-P score, was 11.3 months versus 5.6 months in the enzalutamide and placebo groups (P < .001), and delayed occurrence of first skeletal-related event requiring clinical intervention was also shown.[32,33][Levels of evidence A3 and B1]
- Grade 3 or worse adverse events were more common in the enzalutamide group (43% vs. 37%), primarily because of differences in hypertension, fatigue, and falls. Because patients receiving enzalutamide were on assigned therapy for an average of 1 year longer than those on placebo, the duration of response was longer in patients receiving enzalutamide, and this difference may have contributed to the increase in adverse events.
- Enzalutamide has also been tested in patients with clinically nonmetastatic, hormone-resistant prostate cancer (defined as PSA doubling time ≤10 months while undergoing hormonal therapy).[35]
- In the double-blind phase III PROSPER trial (NCT02003924), 1,401 men without clinical metastases on imaging, but with a rapidly rising PSA, were randomly assigned in a 2:1 ratio to receive either enzalutamide (160 mg PO qd) or placebo. After follow-up of up to 41 months, enzalutamide showed superiority in the primary end point, metastasis-free survival: 77% versus 51% (median 36.6 vs. 14.7 months; HR, 0.29; 95% CI, 0.24–0.35; P < .001).[35][Level of evidence B1]
- OS data were not mature, but at the time of the report, 11% of the men had died in the enzalutamide arm versus 13% in the placebo arm.
- The rate of decline in health-related quality of life was the same in both arms.
- Grade 3 or higher toxicities were more common in the enzalutamide group: 31% versus 23%.
- There were also excesses in several adverse events of special interest because they have been reported previously in patients treated with enzalutamide, including hypertension (12% vs. 5%), major cardiovascular events (5% vs. 3%), and mental impairment disorders (5% vs. 2%).
- Continuing enzalutamide in patients who were switched to abiraterone because of progression, and who had castration-resistant metastatic prostate cancer and a rising PSA while receiving enzalutamide, did not appear to improve the rate of PFS or of clinical progression, a strategy that was tested in the randomized PLATO trial (NCT01995513).[36][Level of evidence B1]
- Apalutamide, an androgen receptor antagonist, has been tested in patients with clinically nonmetastatic, castration-resistant prostate cancer (defined as PSA doubling time ≤10 months while undergoing androgen deprivation therapy).[37] In the trial, 1,207 men were randomly assigned in a 2:1 ratio to receive either daily apalutamide (240 mg PO) or a placebo. All continued their previous ADT.
- With a median follow-up of 20.3 months, metastasis-free survival was 40.5 months in the apalutamide group compared with 16.2 months on placebo (HR, 0.28; 95% CI, 0.23–0.35; P < .001).[37][Level of evidence B1]
- There was a trend toward improved OS in the apalutamide group, but it did not reach statistical significance at the time of the report (HR, 0.70; 95% CI, 0.47–1.04; P = .07).
- There were increases in a number of toxicities associated with apalutamide treatment, which included the following: bone fractures (11.7% vs. 6.5%), hypothyroidism (8.1% vs. 2.0%), fatigue (30.4% vs. 21.1%), hypertension (24.8% vs. 19.8%), rash (23.8% vs. 5.5%), diarrhea (20.3% vs. 15.1%), weight loss (16.1% vs. 6.3%), arthralgias (15.9% vs. 7.5%), and falls (15.6% vs. 9.0%).
- In a prespecified exploratory analysis, QOL over time was similar in the apalutamide and placebo arms, as assessed overall and for all component subscale scores of the FACT-P and EuroQol five-dimension, three-level (EQ-5D-3L) questionnaires.[38][Level of evidence A3]
- Darolutamide, another androgen receptor antagonist, has also been shown to prolong metastasis-free survival and OS in men with nonmetastatic castration-resistant prostate cancer.[39,40] A distinguishing characteristic of darolutamide is its low penetration of the blood-brain barrier. The U.S. Food and Drug Administration approved darolutamide specifically for nonmetastatic, castration-resistant prostate cancer, a more limited label compared with enzalutamide and apalutamide.
A randomized controlled trial included 1,509 men with nonmetastatic castration-resistant prostate cancer, a rising PSA, and a castrate testosterone level. Patients were randomly assigned in a 2:1 ratio to receive darolutamide or placebo while continuing ADT.[39,40]
- The 3-year OS rate was 83% for patients who received darolutamide (95% CI, 80%–86%) and 77% for patients who received placebo (95% CI, 72%–81%).[39,40][Level of evidence A1]
- The darolutamide arm was also associated with longer metastasis-free survival (HR, 0.41; 95% CI, 0.34–0.50).
- Patient-reported QOL was similar in the two arms and differences favored the darolutamide arm. There were statistically significant differences favoring darolutamide for measures of pain, well-being, and urinary symptoms, but the differences did not reach clinically meaningful levels.
- In contrast to studies of enzalutamide and apalutamide, darolutamide was not associated with a higher incidence of falls or fractures, hypertension, or central nervous system–related adverse effects when compared with placebo.
Evidence (hormonal approaches for progressive disease with previous chemotherapy):
- Men with metastatic prostate cancer who had biochemical or clinical progression after treatment with docetaxel (N = 1,195) were randomly assigned in a 2:1 ratio to receive either abiraterone acetate (1,000 mg) (n = 797) or placebo (n = 398) by mouth every day (COU-AA-301 [NCT00638690]). Both groups received prednisone (5 mg PO bid).[41][Level of evidence A1]
- After a median follow-up of 12.8 months, the trial was stopped when an interim analysis showed an OS advantage in the abiraterone group. The final report of the trial was published after a median follow-up of 20.2 months.
- Median OS was 15.8 months in the abiraterone group versus 11.2 months in the placebo group (HRdeath, 0.74; 95% CI, 0.64–0.86; P < .0001).
- Compared with placebo, abiraterone was also associated with a delay in median time to deterioration in the FACT-P QOL score (59.9 weeks vs. 36.1 weeks, P < .0001) and a clinically important improvement in QOL in men with functional status impairment at baseline (48% vs. 32%, P < .0001).[42][Level of evidence A3]
- Enzalutamide has also been shown to increase survival in patients with progressive prostate cancer who previously received ADT as well as docetaxel. In a double-blind, placebo-controlled trial, 1,129 men were randomly assigned in a 2:1 ratio to receive enzalutamide (160 mg PO qd) versus placebo.[43,44,45,46][Levels of evidence A1 and A3]
- After a median follow-up of 14.4 months, the study was stopped at the single-planned interim analysis because improved OS, the primary end point, was found in the enzalutamide study group (median OS, 18.4 months; 95% CI, 17.3–not-yet-reached vs. 13.6 months; 95% CI, 11.3–15.8; HRdeath, 0.63; 95% CI, 0.53–0.75; P < .001). In addition, QOL, measured by the FACT-P questionnaire, was superior in the enzalutamide arm, as was time to first skeletal-related event.[44,46]
- A seizure was reported in 5 of the 800 men in the enzalutamide study group versus none in the placebo group; however, the relationship to enzalutamide is not clear. Of the reported seizures, two patients had brain metastases, one patient had just received intravenous (IV) lidocaine, and one seizure was not witnessed.
Prevention of bone metastases
Painful bone metastases can be a major problem for patients with prostate cancer. Many strategies have been studied for palliation, including the following:[47,48,49,50,51]
- External-beam radiation therapy (EBRT).
- Bone-seeking radionuclides (strontium chloride Sr 89 [89Sr]).
- Denosumab (a monoclonal antibody that inhibits osteoclast function).
- Pain medication.
- Corticosteroids.
- Bisphosphonates.
For more information, see Cancer Pain.
Evidence (palliation for bone metastases using radiation therapy):
- EBRT for palliation of bone pain can be very useful. A single fraction of 8 Gy has been shown to have similar benefits on bone pain relief and QOL as multiple fractions (3 Gy × 10) was seen in the RTOG-9714 trial (NCT00003162), for example.[52,53][Level of evidence A3]
Evidence (palliation for bone metastases using strontium chloride):
The use of radioisotopes such as 89Sr has been effective as palliative treatment of some patients with osteoblastic metastases. As a single agent, 89Sr has been reported to decrease bone pain in 80% of patients treated.[54]
- A multicenter randomized trial of a single IV dose of 89Sr (150 MBq: 4 mCi) versus palliative EBRT was done in men with painful bone metastases from prostate cancer despite hormone treatment.[55][Level of evidence A1]; [56]
- Similar subjective pain response rates were shown in both groups: 34.7% for 89Sr versus 33.3% for EBRT alone.
- OS was better in the EBRT group than in the 89Sr group (P = .046; median survival, 11.0 months vs. 7.2 months).
- No statistically significant differences in time to subjective progression or in PFS were seen.
- When used as an adjunct to EBRT, 89Sr was shown to slow disease progression and to reduce analgesic requirements, compared with EBRT alone.
Evidence (palliation or prevention of bone metastases using denosumab):
- A placebo-controlled randomized trial (NCT00321620) compared denosumab with zoledronic acid for the prevention of skeletal events (pathological fractures, spinal cord compression, or the need for palliative bone radiation or surgery) in men with hormonal therapy-resistant prostate cancer with at least one bone metastasis.[47]
- The trial reported that denosumab was more effective than zoledronic acid; median time to first on-study skeletal event was 20.7 versus 17.1 months (HR, 0.82; 95% CI, 0.71–0.95).
- Serious adverse events were reported in 63% of denosumab patients versus 60% in patients on zoledronic acid. The cumulative incidence of osteonecrosis of the jaw was low in both study arms (2% in the denosumab arm vs. 1% in the zoledronic acid arm). There was grade 3 to 4 toxicity. There was no difference in survival. The incidence of hypocalcemia was higher in the denosumab arm (13% vs. 6%).[57]
- A randomized placebo-controlled trial included 1,432 men with castration-resistant prostate cancer with no evidence of any metastases who were given denosumab (120 mg administered subcutaneously every 4 weeks) to prevent the first evidence of bone metastasis (whether symptomatic or not).[57][Level of evidence B1]
- After a median follow-up of 20 months, median bone metastasis-free survival was 29.5 versus 25.2 months in the denosumab versus placebo arms (HR, 0.85; 95% CI, 0.73–0.98; P = .028).
- Symptomatic bone metastases were reported in 69 (10%) denosumab patients versus 96 (13%) placebo patients (HR, 0.67; 95% CI, 0.49–0.92; P = .01).
- There were no differences in OS between the two groups.
- Osteonecrosis occurred in 33 (5%) of men on the denosumab arm versus none on the placebo arm. Hypocalcemia occurred in 12 (2%) versus 2 (<1%) men, and urinary retention in 54 (8%) of men on denosumab versus 31 (4%) of men on placebo.
Treatment Options for Recurrent Prostate Cancer
Treatment options for patients with recurrent prostate cancer include the following:
- Hormone therapy.
- Chemotherapy for hormone-resistant prostate cancer.
- Immunotherapy.
- Radiopharmaceutical therapy.
- Poly (ADP-ribose) polymerase (PARP) inhibitors for men with prostate cancer and BRCA1, BRCA2, and/or ATM mutations.
Chemotherapy for hormone-sensitive or hormone-resistant prostate cancer
Evidence (chemotherapy for hormone-sensitive or hormone-resistant prostate cancer):
- A randomized trial showed improved pain control in patients with hormone-resistant prostate cancer treated with mitoxantrone plus prednisone compared with those treated with prednisone alone.[58] Differences in OS or measured global QOL between the two treatments were not statistically significant.
- Docetaxel has been shown to improve OS compared with mitoxantrone. In a randomized trial involving patients with hormone-refractory prostate cancer, docetaxel (75 mg/m2 every 3 weeks) and docetaxel (30 mg/m2 weekly for 5 out of every 6 weeks) were compared with mitoxantrone (12 mg/m2 every 3 weeks). All patients received oral prednisone (5 mg bid). Patients in the docetaxel arms also received high-dose dexamethasone pretreatment for each docetaxel administration (8 mg given at 12 hours, 3 hours, and 1 hour before the 3-week regimen; 8 mg given at 1 hour before the 5 out-of-every-6 weeks' regimen).[59]
- OS at 3 years was statistically significantly better in the 3-weekly docetaxel arm (18.6%) than in the mitoxantrone arm (13.5%, HRdeath, 0.79; 95% CI, 0.67–0.93).
- However, the OS rate for the 5 out-of-every-6 weeks docetaxel regimen was 16.8%, which was not statistically significantly better than mitoxantrone.
- QOL was also superior in the docetaxel arms compared with mitoxantrone (P = .009).[60][Levels of evidence A1 and A3]
- In another randomized trial involving patients with hormone-refractory prostate cancer, a 3-week regimen of estramustine (280 mg PO tid for days 1 to 5, plus daily warfarin and 325 mg aspirin to prevent vascular thrombosis), and docetaxel (60 mg/m2 IV on day 2, preceded by dexamethasone [20 mg × 3 starting the night before]) was compared with mitoxantrone (12 mg/m2 IV every 3 weeks) plus prednisone (5 mg qd).[61][Level of evidence A1]
- After a median follow-up of 32 months, median OS was 17.5 months in the estramustine/docetaxel arm versus 15.6 months in the mitoxantrone arm (HRdeath, 0.80; 95% CI, 0.67–0.97; P = .02).
- Global QOL and pain palliation measures were similar in the two treatment arms.[62][Level of evidence A3]
- A 2-weekly regimen of docetaxel has been compared with a 3-weekly regimen. OS appeared to be better in the 2-week regimen, and hematologic toxicity was less.[63][Level of evidence A1]
- In the trial, 361 men with metastatic hormone-resistant prostate cancer were randomly assigned to receive docetaxel either in a 2-weekly regimen (50 mg/m2 IV) or a 3-weekly regimen (75 mg/m2 IV) until progression. All patients were also to receive prednisolone (10 mg PO qd) and dexamethasone (7.5–8.0 mg qd), starting the day before and continuing for 1 to 2 days after each docetaxel dose. Fifteen randomly assigned patients (4.2%) were deemed ineligible in retrospect or withdrew consent, and they were dropped from the analysis.
- With a median follow-up of 18 months, there was a small difference in time to treatment failure, the primary end point of the study (5.6 months [95% CI, 5.0–6.2] vs. 4.9 months [95% CI, 4.5–5.4]; P = .014). However, there was a larger difference in median OS, a secondary end point, in favor of the 2-week regimen (19.5 months [95% CI, 15.9–23.1] vs. 17.0 months [95% CI, 15.0 –19.1]; P = .02).
- There was a lower rate of grade 3 to 4 neutropenia with the 2-week regimen (36% vs. 53%; P < .0001) and a lower rate of febrile neutropenia (4% vs. 14%; P = .001).
- In patients with metastatic hormone/castrate-refractory prostate cancer (mCRPC) and no previous chemotherapy, cabazitaxel and docetaxel appeared to provide similar results with respect to OS.[64]
- In the FIRSTANA trial (NCT01308567), 1,168 men with mCRPC were randomly assigned in a 1:1:1 ratio to receive cabazitaxel 20 mg/m2, cabazitaxel 25 mg/m2, or docetaxel 75 mg/m2 IV every 3 weeks (plus prednisone 10 mg PO qd) until disease progression. Median OS was similar across all three study arms and not statistically significantly different (24.5 vs. 25.2 vs. 24.3 months, respectively), with virtually overlapping survival curves.[64][Level of evidence A1]
- However, toxicities varied across the study arms, with adverse event rates of 41.2%, 60.1%, and 46.0%, respectively, which required urgent treatment.
- In patients with mCRPC whose disease progressed during or after treatment with docetaxel, cabazitaxel was shown to improve survival compared with mitoxantrone in a randomized trial (NCT00417079).[65] In this trial, 755 such men were treated with prednisone (10 mg PO qd) and randomly assigned to receive either cabazitaxel (25 mg/m2 IV) or mitoxantrone (12 mg/m2 IV) every 3 weeks.[65][Level of evidence A1]
- Median OS was 15.1 months in the cabazitaxel arm and 12.7 months in the mitoxantrone study arm (HRdeath, 0.70; 95% CI, 0.59–0.83; P < .0001).
- In a noninferiority-design randomized trial comparing cabazitaxel (20 mg/m2 IV every 3 weeks) with cabazitaxel (25 mg/m2 IV every 3 weeks) in a similar population of 1,200 men with mCRPC who had received previous docetaxel, the lower dose of cabazitaxel fulfilled noninferiority criteria with respect to OS (HRdeath, 1.024; CI, upper bound at 1.184), but with less toxicity.[66][Level of evidence A1]
Other chemotherapy regimens reported to produce subjective improvement in symptoms and reduction in PSA level include the following:[67][Level of evidence C2]; [68]
- Paclitaxel.
- Estramustine/etoposide.
- Estramustine/vinblastine.
- Estramustine/paclitaxel.
A study suggests that patients whose tumors exhibit neuroendocrine differentiation are more responsive to chemotherapy.[69]
Immunotherapy
Sipuleucel-T, an active cellular immunotherapy, has been shown to increase OS in patients with hormone-refractory metastatic prostate cancer. Sipuleucel-T consists of autologous peripheral blood mononuclear cells that have been exposed ex vivo to a recombinant fusion protein (PA2024) composed of prostatic acid phosphatase fused to granulocyte-macrophage colony-stimulating factor.
Side effects are generally consistent with cytokine release and include chills, fever, headache, myalgia, sweating, and influenza-like symptoms, usually within the first 24 hours of infusion. No increase in autoimmune disorders or secondary malignancies has been noted.[70]
Evidence (immunotherapy):
- In the largest trial (Immunotherapy for Prostate Adenocarcinoma Treatment: IMPACT trial [NCT00065442]), 512 patients with hormone-refractory metastatic disease were randomly assigned in a 2:1 ratio to receive sipuleucel-T (n = 341) versus placebo (n = 171) by IV in a 60-minute infusion every 2 weeks for a total of 3 doses.[71] Patients with visceral metastases, pathological bone fractures, or ECOG performance status worse than 0–1 were excluded from the study. At documented disease progression, patients in the placebo group could receive, at the physician's discretion, infusions manufactured with the same specifications as sipuleucel-T but using cells that had been cryopreserved at the time that the placebo was prepared (63.7% of the placebo patients received these transfusions). Time to disease progression and time to development of disease-related pain were the initial primary end points, but the primary end point was changed before unblinding based upon survival differences in two previous trials of similar design (described below).[71][Level of evidence A1]
- After a median follow-up of 34.1 months, the overall mortality was 61.6% in the sipuleucel-T group versus 70.8% in the placebo group (HRdeath, 0.78; 95% CI, 0.61–0.98; P = .03). However, the improved survival was not accompanied by measurable antitumor effects.
- There was no difference between the study groups in rate of disease progression. In 2011, the estimated price of sipuleucel-T was $93,000 for a 1-month course of therapy. This translates into an estimated cost of about $276,000 per year-of-life saved.[72]
- The same investigators previously performed two smaller trials (D9901 and D9902A [NCT00005947]) of nearly identical design to the IMPACT trial.[73,74]
- The combined results of the two smaller trials, involving a total of 225 patients randomly assigned in a 2:1 ratio of sipuleucel-T to placebo were like those in the IMPACT trial. The HRdeath was 0.67 (95% CI, 0.49–0.91), but the time-to-progression rates were not statistically significantly different.
Low-dose prednisone may palliate symptoms in some patients.[75]
Evidence (low-dose prednisone for palliation):
- A randomized comparison of prednisone (5 mg qid) with flutamide (250 mg tid) was conducted in patients with disease progression after androgen ablative therapy (castration or LH-RH agonist).[76]
- Prednisone and flutamide produced similar OS, symptomatic response, PSA response, and time to progression; however, there were statistically significant differences in pain, nausea and vomiting, and diarrhea in patients who received prednisone. For more information, see Cancer Pain, Nausea and Vomiting Related to Cancer Treatment, and Gastrointestinal Complications.
Ongoing clinical trials continue to explore the value of chemotherapy for these patients.[10,11,12,13,58,67,68,69]
Radiopharmaceutical therapy
Alpha emitter radiation
Radium Ra 223 (223Ra) emits alpha particles (i.e., two protons and two neutrons bound together, identical to a helium nucleus) with a half-life of 11.4 days. It is administered by IV and selectively taken up by newly formed bone stroma. The high-energy alpha particles have a short range of <100 mcM. 223Ra improved OS in patients with prostate cancer metastatic to the bone.
Evidence (alpha emitter radiation):
- In a placebo-controlled trial, 921 men with symptomatic castration-resistant prostate cancer, two or more bone metastases, and no known visceral metastases, were randomly assigned in a 2:1 ratio to receive 223Ra at a dose of 50kBq per kg body weight every 4 weeks for six injections versus placebo. All study participants had already received docetaxel, were not healthy enough to receive it, or declined it.[77,78]
- Median OS was 14.9 months in the 223Ra study group versus 11.3 months in the placebo groups (HRmortality, 0.70; 95% CI, 0.58–0.83; P < .001).[77][Level of evidence A1]
- The rates of symptomatic skeletal events (33% vs. 38%) and spinal cord compression (4% vs. 7%) were also statistically significantly improved.
- Prospectively measured, QOL was also better in the 223Ra study group (25% vs. 16% had a ≥10 point improvement on a scale of 0 to 156; P = .02).[77][Level of evidence A3]
- With administration of 223Ra at a dose of 50kBq per kg of body weight every 4 weeks for 6 injections, the side effects were like those of a placebo.
PARP inhibitors for men with prostate cancer andBRCA1,BRCA2, and/orATMmutations
Olaparib
Evidence (olaparib):
- The PARP inhibitor olaparib was tested in an open-label, phase III, randomized controlled trial in men with metastatic castration-resistant prostate cancer and mutations in one of 15 prespecified genes related to homologous recombination repair, including BRCA1 or BRCA2. The men had previously received enzalutamide, abiraterone, or both, with or without previous taxane chemotherapy.[79] The trial enrolled 387 men and randomly assigned them in a 2:1 ratio to receive olaparib (300 mg twice daily) or physician's choice of enzalutamide or abiraterone plus prednisone.
Cohort A included 245 patients with at least one mutation in BRCA1, BRCA2, or ATM. Cohort B included 142 patients with at least one mutation in one of the other 12 prespecified genes.
- The median OS in cohort A was 19.1 months for patients who received olaparib and 14.7 months for patients who received the control regimen (HR, 0.69; 95% CI, 0.50–0.97). A sensitivity analysis adjusting for crossover to olaparib in the control arm reported an HR of 0.42 (95% CI, 0.19–0.91).
- There was no significant OS benefit in cohort B.
- There was no significant OS benefit in the overall population.
- The most common adverse events for patients who received olaparib were anemia (50%), nausea (43%), and fatigue or asthenia (42%).
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
-
Trock BJ, Han M, Freedland SJ, et al.: Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299 (23): 2760-9, 2008.
-
Ray GR, Bagshaw MA, Freiha F: External beam radiation salvage for residual or recurrent local tumor following radical prostatectomy. J Urol 132 (5): 926-30, 1984.
-
Carter GE, Lieskovsky G, Skinner DG, et al.: Results of local and/or systemic adjuvant therapy in the management of pathological stage C or D1 prostate cancer following radical prostatectomy. J Urol 142 (5): 1266-70; discussion 1270-1, 1989.
-
Freeman JA, Lieskovsky G, Cook DW, et al.: Radical retropubic prostatectomy and postoperative adjuvant radiation for pathological stage C (PcN0) prostate cancer from 1976 to 1989: intermediate findings. J Urol 149 (5): 1029-34, 1993.
-
Shipley WU, Seiferheld W, Lukka HR, et al.: Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med 376 (5): 417-428, 2017.
-
Moul JW, Paulson DF: The role of radical surgery in the management of radiation recurrent and large volume prostate cancer. Cancer 68 (6): 1265-71, 1991.
-
Schellhammer PF, Kuban DA, el-Mahdi AM: Treatment of clinical local failure after radiation therapy for prostate carcinoma. J Urol 150 (6): 1851-5, 1993.
-
Bales GT, Williams MJ, Sinner M, et al.: Short-term outcomes after cryosurgical ablation of the prostate in men with recurrent prostate carcinoma following radiation therapy. Urology 46 (5): 676-80, 1995.
-
Taylor CD, Elson P, Trump DL: Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol 11 (11): 2167-72, 1993.
-
Debruyne FJ, Murray R, Fradet Y, et al.: Liarozole--a novel treatment approach for advanced prostate cancer: results of a large randomized trial versus cyproterone acetate. Liarozole Study Group. Urology 52 (1): 72-81, 1998.
-
Eisenberger MA: Chemotherapy for prostate carcinoma. NCI Monogr (7): 151-63, 1988.
-
Pienta KJ, Redman B, Hussain M, et al.: Phase II evaluation of oral estramustine and oral etoposide in hormone-refractory adenocarcinoma of the prostate. J Clin Oncol 12 (10): 2005-12, 1994.
-
Hudes GR, Greenberg R, Krigel RL, et al.: Phase II study of estramustine and vinblastine, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol 10 (11): 1754-61, 1992.
-
Kuban DA, el-Mahdi AM, Schellhammer PF: Prostate-specific antigen for pretreatment prediction and posttreatment evaluation of outcome after definitive irradiation for prostate cancer. Int J Radiat Oncol Biol Phys 32 (2): 307-16, 1995.
-
Sandler HM, Dunn RL, McLaughlin PW, et al.: Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys 48 (3): 629-33, 2000.
-
Duchesne GM, Woo HH, Bassett JK, et al.: Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol 17 (6): 727-37, 2016.
-
Duchesne GM, Woo HH, King M, et al.: Health-related quality of life for immediate versus delayed androgen-deprivation therapy in patients with asymptomatic, non-curable prostate cancer (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol 18 (9): 1192-1201, 2017.
-
Crook JM, O'Callaghan CJ, Duncan G, et al.: Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med 367 (10): 895-903, 2012.
-
Magnan S, Zarychanski R, Pilote L, et al.: Intermittent vs Continuous Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 1 (9): 1261-9, 2015.
-
Freedland SJ, de Almeida Luz M, De Giorgi U, et al.: Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 389 (16): 1453-1465, 2023.
-
Kunath F, Grobe HR, Rücker G, et al.: Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev (6): CD009266, 2014.
-
Fosså SD, Dearnaley DP, Law M, et al.: Prognostic factors in hormone-resistant progressing cancer of the prostate. Ann Oncol 3 (5): 361-6, 1992.
-
Kelly WK, Scher HI, Mazumdar M, et al.: Prostate-specific antigen as a measure of disease outcome in metastatic hormone-refractory prostate cancer. J Clin Oncol 11 (4): 607-15, 1993.
-
Small EJ, Vogelzang NJ: Second-line hormonal therapy for advanced prostate cancer: a shifting paradigm. J Clin Oncol 15 (1): 382-8, 1997.
-
Small EJ, Halabi S, Dawson NA, et al.: Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol 22 (6): 1025-33, 2004.
-
Sridhara R, Eisenberger MA, Sinibaldi VJ, et al.: Evaluation of prostate-specific antigen as a surrogate marker for response of hormone-refractory prostate cancer to suramin therapy. J Clin Oncol 13 (12): 2944-53, 1995.
-
Hussain M, Wolf M, Marshall E, et al.: Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: a Southwest Oncology Group report. J Clin Oncol 12 (9): 1868-75, 1994.
-
Sternberg CN, Castellano D, Daugaard G, et al.: Abiraterone acetate for patients with metastatic castration-resistant prostate cancer progressing after chemotherapy: final analysis of a multicentre, open-label, early-access protocol trial. Lancet Oncol 15 (11): 1263-8, 2014.
-
Ryan CJ, Smith MR, de Bono JS, et al.: Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368 (2): 138-48, 2013.
-
Ryan CJ, Smith MR, Fizazi K, et al.: Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16 (2): 152-60, 2015.
-
Basch E, Autio K, Ryan CJ, et al.: Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol 14 (12): 1193-9, 2013.
-
Beer TM, Armstrong AJ, Rathkopf DE, et al.: Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371 (5): 424-33, 2014.
-
Loriot Y, Miller K, Sternberg CN, et al.: Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol 16 (5): 509-21, 2015.
-
Beer TM, Armstrong AJ, Rathkopf D, et al.: Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol 71 (2): 151-154, 2017.
-
Hussain M, Fizazi K, Saad F, et al.: Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 378 (26): 2465-2474, 2018.
-
Attard G, Borre M, Gurney H, et al.: Abiraterone Alone or in Combination With Enzalutamide in Metastatic Castration-Resistant Prostate Cancer With Rising Prostate-Specific Antigen During Enzalutamide Treatment. J Clin Oncol 36 (25): 2639-2646, 2018.
-
Smith MR, Saad F, Chowdhury S, et al.: Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 378 (15): 1408-1418, 2018.
-
Saad F, Cella D, Basch E, et al.: Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol 19 (10): 1404-1416, 2018.
-
Fizazi K, Shore N, Tammela TL, et al.: Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 380 (13): 1235-1246, 2019.
-
Fizazi K, Shore N, Tammela TL, et al.: Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med 383 (11): 1040-1049, 2020.
-
de Bono JS, Logothetis CJ, Molina A, et al.: Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364 (21): 1995-2005, 2011.
-
Harland S, Staffurth J, Molina A, et al.: Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer 49 (17): 3648-57, 2013.
-
Scher HI, Fizazi K, Saad F, et al.: Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367 (13): 1187-97, 2012.
-
Fizazi K, Scher HI, Miller K, et al.: Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol 15 (10): 1147-56, 2014.
-
Sternberg CN, de Bono JS, Chi KN, et al.: Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial. Ann Oncol 25 (2): 429-34, 2014.
-
Cella D, Ivanescu C, Holmstrom S, et al.: Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol 26 (1): 179-85, 2015.
-
Fizazi K, Carducci M, Smith M, et al.: Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377 (9768): 813-22, 2011.
-
Scher HI, Chung LW: Bone metastases: improving the therapeutic index. Semin Oncol 21 (5): 630-56, 1994.
-
Dearnaley DP, Sydes MR, Mason MD, et al.: A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 95 (17): 1300-11, 2003.
-
Ernst DS, Tannock IF, Winquist EW, et al.: Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol 21 (17): 3335-42, 2003.
-
Saad F, Gleason DM, Murray R, et al.: Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96 (11): 879-82, 2004.
-
Kaasa S, Brenne E, Lund JA, et al.: Prospective randomised multicenter trial on single fraction radiotherapy (8 Gy x 1) versus multiple fractions (3 Gy x 10) in the treatment of painful bone metastases. Radiother Oncol 79 (3): 278-84, 2006.
-
Chow E, Harris K, Fan G, et al.: Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 25 (11): 1423-36, 2007.
-
Robinson RG: Strontium-89--precursor targeted therapy for pain relief of blastic metastatic disease. Cancer 72 (11 Suppl): 3433-5, 1993.
-
Oosterhof GO, Roberts JT, de Reijke TM, et al.: Strontium(89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: a phase III study of the European Organisation for Research and Treatment of Cancer, Genitourinary Group. Eur Urol 44 (5): 519-26, 2003.
-
Porter AT, McEwan AJ, Powe JE, et al.: Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 25 (5): 805-13, 1993.
-
Smith MR, Saad F, Coleman R, et al.: Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379 (9810): 39-46, 2012.
-
Tannock IF, Osoba D, Stockler MR, et al.: Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 14 (6): 1756-64, 1996.
-
Tannock IF, de Wit R, Berry WR, et al.: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351 (15): 1502-12, 2004.
-
Berthold DR, Pond GR, Soban F, et al.: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 26 (2): 242-5, 2008.
-
Petrylak DP, Tangen CM, Hussain MH, et al.: Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351 (15): 1513-20, 2004.
-
Berry DL, Moinpour CM, Jiang CS, et al.: Quality of life and pain in advanced stage prostate cancer: results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. J Clin Oncol 24 (18): 2828-35, 2006.
-
Kellokumpu-Lehtinen PL, Harmenberg U, Joensuu T, et al.: 2-Weekly versus 3-weekly docetaxel to treat castration-resistant advanced prostate cancer: a randomised, phase 3 trial. Lancet Oncol 14 (2): 117-24, 2013.
-
Oudard S, Fizazi K, Sengeløv L, et al.: Cabazitaxel Versus Docetaxel As First-Line Therapy for Patients With Metastatic Castration-Resistant Prostate Cancer: A Randomized Phase III Trial-FIRSTANA. J Clin Oncol 35 (28): 3189-3197, 2017.
-
de Bono JS, Oudard S, Ozguroglu M, et al.: Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376 (9747): 1147-54, 2010.
-
Eisenberger M, Hardy-Bessard AC, Kim CS, et al.: Phase III Study Comparing a Reduced Dose of Cabazitaxel (20 mg/m(2)) and the Currently Approved Dose (25 mg/m(2)) in Postdocetaxel Patients With Metastatic Castration-Resistant Prostate Cancer-PROSELICA. J Clin Oncol 35 (28): 3198-3206, 2017.
-
Petrylak DP, Macarthur RB, O'Connor J, et al.: Phase I trial of docetaxel with estramustine in androgen-independent prostate cancer. J Clin Oncol 17 (3): 958-67, 1999.
-
Millikan RE: Chemotherapy of advanced prostatic carcinoma. Semin Oncol 26 (2): 185-91, 1999.
-
Amato RJ, Logothetis CJ, Hallinan R, et al.: Chemotherapy for small cell carcinoma of prostatic origin. J Urol 147 (3 Pt 2): 935-7, 1992.
-
Hall SJ, Klotz L, Pantuck AJ, et al.: Integrated safety data from 4 randomized, double-blind, controlled trials of autologous cellular immunotherapy with sipuleucel-T in patients with prostate cancer. J Urol 186 (3): 877-81, 2011.
-
Kantoff PW, Higano CS, Shore ND, et al.: Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363 (5): 411-22, 2010.
-
Longo DL: New therapies for castration-resistant prostate cancer. N Engl J Med 363 (5): 479-81, 2010.
-
Higano CS, Schellhammer PF, Small EJ, et al.: Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115 (16): 3670-9, 2009.
-
Small EJ, Schellhammer PF, Higano CS, et al.: Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24 (19): 3089-94, 2006.
-
Tannock I, Gospodarowicz M, Meakin W, et al.: Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 7 (5): 590-7, 1989.
-
Fosså SD, Slee PH, Brausi M, et al.: Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European organization for research and treatment of cancer genitourinary group. J Clin Oncol 19 (1): 62-71, 2001.
-
Parker C, Nilsson S, Heinrich D, et al.: Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369 (3): 213-23, 2013.
-
Sartor O, Coleman R, Nilsson S, et al.: Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 15 (7): 738-46, 2014.
-
Hussain M, Mateo J, Fizazi K, et al.: Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 383 (24): 2345-2357, 2020.
Key References for Prostate Cancer
These references have been identified by members of the PDQ Adult Treatment Editorial Board as significant in the field of prostate cancer treatment. This list is provided to inform users of important studies that have helped shape the current understanding of and treatment options for prostate cancer. Listed after each reference are the sections within this summary where the reference is cited.
- Beer TM, Armstrong AJ, Rathkopf DE, et al.: Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371 (5): 424-33, 2014. [PUBMED Abstract]
Cited in:
- Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
- Berthold DR, Pond GR, Soban F, et al.: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 26 (2): 242-5, 2008. [PUBMED Abstract]
Cited in:
- Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
- Bill-Axelson A, Holmberg L, Garmo H, et al.: Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 370 (10): 932-42, 2014. [PUBMED Abstract]
Cited in:
- Treatment Option Overview for Prostate Cancer
- Treatment of Stage II Prostate Cancer
- de Bono JS, Oudard S, Ozguroglu M, et al.: Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376 (9747): 1147-54, 2010. [PUBMED Abstract]
Cited in:
- Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
- Lu-Yao GL, Albertsen PC, Moore DF, et al.: Outcomes of localized prostate cancer following conservative management. JAMA 302 (11): 1202-9, 2009. [PUBMED Abstract]
Cited in:
- General Information About Prostate Cancer
- Treatment Option Overview for Prostate Cancer
- Mason MD, Parulekar WR, Sydes MR, et al.: Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. J Clin Oncol 33 (19): 2143-50, 2015. [PUBMED Abstract]
Cited in:
- Treatment of Stage II Prostate Cancer
- Treatment of Stage III Prostate Cancer
- Parker C, Nilsson S, Heinrich D, et al.: Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369 (3): 213-23, 2013. [PUBMED Abstract]
Cited in:
- Treatment Option Overview for Prostate Cancer
- Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
- Potosky AL, Davis WW, Hoffman RM, et al.: Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst 96 (18): 1358-67, 2004. [PUBMED Abstract]
Cited in:
- Treatment Option Overview for Prostate Cancer
- Pound CR, Partin AW, Eisenberger MA, et al.: Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281 (17): 1591-7, 1999. [PUBMED Abstract]
Cited in:
- General Information About Prostate Cancer
- Ryan CJ, Smith MR, Fizazi K, et al.: Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16 (2): 152-60, 2015. [PUBMED Abstract]
Cited in:
- Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
- Scher HI, Fizazi K, Saad F, et al.: Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367 (13): 1187-97, 2012. [PUBMED Abstract]
Cited in:
- Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
- Seidenfeld J, Samson DJ, Aronson N, et al.: Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid Rep Technol Assess (Summ) (4): I-x, 1-246, I1-36, passim, 1999. [PUBMED Abstract]
Cited in:
- Treatment of Stage I Prostate Cancer
- Treatment of Stage II Prostate Cancer
- Treatment of Stage III Prostate Cancer
- Treatment of Stage IV Prostate Cancer
- Sternberg CN, Castellano D, Daugaard G, et al.: Abiraterone acetate for patients with metastatic castration-resistant prostate cancer progressing after chemotherapy: final analysis of a multicentre, open-label, early-access protocol trial. Lancet Oncol 15 (11): 1263-8, 2014. [PUBMED Abstract]
Cited in:
- Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
- Thompson IM, Tangen CM, Paradelo J, et al.: Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181 (3): 956-62, 2009. [PUBMED Abstract]
Cited in:
- Treatment of Stage I Prostate Cancer
- Treatment of Stage II Prostate Cancer
- Treatment of Stage III Prostate Cancer
- Wilt TJ, Brawer MK, Jones KM, et al.: Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 367 (3): 203-13, 2012. [PUBMED Abstract]
Cited in:
- General Information About Prostate Cancer
- Treatment Option Overview for Prostate Cancer
- Treatment of Stage I Prostate Cancer
- Treatment of Stage II Prostate Cancer
Latest Updates to This Summary (08 / 09 / 2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment of Recurrent Hormone-Sensitive or Hormone-Resistant Prostate Cancer
This section was renamed from Treatment of Recurrent or Hormone-Resistant Prostate Cancer.
Added Nonsteroidal antiandrogen therapy with or without androgen deprivation therapy as a new subsection.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of prostate cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Prostate Cancer Treatment are:
- Juskaran S. Chadha, DO (Moffitt Cancer Center)
- Timothy Gilligan, MD (Cleveland Clinic Taussig Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Prostate Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/prostate/hp/prostate-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389471]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us.
Last Revised: 2024-08-09